1. Introduction
Coronavirus disease 2019 (COVID-19) started in December 2019. Although the typical clinical picture is mainly related to the respiratory system, including fever, shortness of breath and cough, but there is ongoing evidence that other body systems might be affected. For example, neurological disorders have also been reported. Different cases of COVID-19 associated with acute necrotizing hemorrhagic encephalopathy (1), meningoencephalitis complicated with intracranial hemorrhage (2), stroke, febrile seizures, and acute peripheral nerve disease (3, 4) have been introduced previously. Here, a case of Guillain-Barre syndrome (GBS) following COVID-19 is described.
2. Case Presentation
A 66-year-old female patient with a medical history of back and shoulder pain was admitted to the neurological department, Golestan Hospital, on August 23, 2020, with misbalance and acute weakness of upper and lower limbs with preference of the proximal parts of the limbs and pinprick from 4 days ago. In addition, she showed a loss of deep tendon reflexes and was unable to sit, stand or walk and could not raise her hands and hold objects on the day of hospitalization. It is worth mentioning that the patient reported headache, fever, and cough two weeks before admission and on August 17 (6 days before admission), she was found with positive coronavirus antibodies (Ig G and Ig M) in serologic testing. One day after admission, she had a negative result for SARS-CoV-2 RNA in nasal swabs.
Although she did not experience fever and gastrointestinal symptoms after admission and standard laboratory tests (complete blood count, C-reactive protein (CRP), serum glucose, and urine tests) showed the normal range, the chest X-ray showed the lung infection. Taken together, these results confirmed that she had been infected by SARS-CoV-2 previously. According to the patient's symptoms, the GBS was diagnosed.
The patient was awake, and the facial sense, pupil response to the light, eye movements and hearing were symmetrical and normal, but she showed a mild delirium due to problems with breathing (verified by CT scan), which was related to the lung infection. In addition, the lumbar puncture (LP) was done and cerebrospinal fluid (CSF) analysis showed the red blood cell (RBC) count of 5 and glucose level of 110 mg/dL (vs. 150 -160 mg/dL blood glucose). In addition, PCR results showed the presence of SARS-CoV-2 RNA.
Magnetic resonance imaging (MRI) was performed and there was no evidence of hemorrhage, hydrocephalous and white and gray matter edema. In addition, it revealed the increased scattered signals in white matter, basal ganglia and pons in favor of small vessel disease.
Four days after admission, the patient showed the worsening of the GBS symptoms. Therefore, plasmapheresis was given over the course of 8 days with a volume of 2000 mL (on every alternate day), which was followed by the recovery of the upper and lower limbs powers from 2/5 to 4/5 and the improvement of the neck flexor muscles from 3/5 to 5/5.
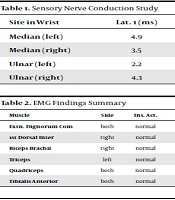
Follow-up electrophysiological study was performed 20 days after admission. The electromyogram (EMG)- nerve conduction velocity (NCV) results revealed motor and sensory neuropathy, which was generally accompanied by demyelination (Table 1, 2 and 3).
| Site in Wrist | Lat. 1 (ms) | Lat. 2 (ms) | Amp (uV) | Area (uVms) | Distance (mm) | Interval (ms) | NCV (m/s) |
|---|---|---|---|---|---|---|---|
| Median (left) | 4.9 | 5.9 | 10.5 | 0.7 | 130 | 4.9 | 26.6 |
| Median (right) | 3.5 | 4.5 | 8.2 | 0.4 | 130 | 3.5 | 37.6 |
| Ulnar (left) | 2.2 | 2.6 | 5.7 | 0.1 | 110 | 2.2 | 50.9 |
| Ulnar (right) | 4.3 | 5.5 | 6.0 | 0.7 | 110 | 4.3 | 25.7 |
Sensory Nerve Conduction Study
| Muscle | Side | Ins. Act. | Fibs. | Pos. Wave | Fasc. | MYO.Disch. | Normal MUP | Poly | Low Amp. | High Amp. | Dur. | Recruit | Int. Patt. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extn. Digitorum Com | both | normal | 0 | 0 | 0 | 0 | +3 | +1 | 0 | +1 | normal | full | full |
| 1st Dorsal Inter | right | normal | 0 | 0 | 0 | 0 | +4 | N | 0 | 0 | normal | full | full |
| Biceps Brachii | right | normal | 0 | +2 | 0 | 0 | +4 | N | 0 | +1 | normal | full | full |
| Triceps | left | normal | +1 | 0 | 0 | 0 | +4 | N | 0 | 0 | normal | full | full |
| Quadriceps | both | normal | +1 | +1 | 0 | 0 | +4 | N | 0 | 0 | normal | full | full |
| Tibialis Anterior | both | normal | 0 | 0 | 0 | 0 | +3 | +1 | 0 | +2 | normal | reduce | full |
EMG Findings Summary
| Nerve | Side | Stim. Site | F- Lat. (ms) | M - Lat. (ms) | F - M Lat. (ms) | F- Occurr |
|---|---|---|---|---|---|---|
| Ulnar | left | wrist | 35.0 | 2.5 | 32.5 | 10 /15 ,67 |
| Ulnar | right | wrist | 28.6 | 10 /10 ,100 | ||
| Tibial | left | ankle | 55.6 | 10 /10 ,100 | ||
| Tibial | right | ankle | 57.6 | 10 /10 ,100 |
F-Waves of the Case
3. Discussion
Neuropathic features of COVID-19 have been previously described in different cases. It was reported that the SARS-CoV-2 enters the central nervous system (CNS) through the olfactory bulb following nasal infection and subsequently causes inflammation and demyelination. Moreover, it was reported that the virus without inducing fever and typical respiratory or gastrointestinal symptoms in infected patients can lead to transitory loss of smell and taste (4). Some COVID-19 patients were found with respiratory distress syndrome and neurological symptoms caused by encephalopathy with agitation, confusion, dysexecutive syndrome, ataxia and corticospinal tract signs (3). GBS is an acute/subacute immune-mediated polyradiculoneuropathy, which is characterized by varying degrees of limbs or cranial-nerves weakness, loss of deep tendon reflexes and sensory and dysautonomic symptoms due to demyelination of the peripheral nerves and their roots and/or axonal damage (5). The GBS includes several pathological subtypes, and the most common type is a multifocal demyelinating disorder of the peripheral nerves, which is related to macrophages. Immunological studies showed that at least one-third of GBS patients produce antibodies against nerve gangliosides, which can react with constituents of the liposaccharide of Campylobacter jejuni. In the Miller Fisher variant of the disease, these antiganglioside antibodies can lead to neuromuscular block and may in part explain the clinical signs of that disorder (6).
Electrodiagnostic characteristics of this current case are in line with other reports, in which it was reported that GBS patients encompass signs of segmental demyelination as temporal dispersion of the compound muscle action potentials and sensory potentials, prolonged distal motor latencies, reduced conduction velocity in the demyelination range, conduction blocks and the absence of or prolonged F waves (5). The symptoms of the current case confirm the recent observations, indicating that two-third of all GBS patients are preceded by upper respiratory infection (5) and previous respiratory syndrome in COVID-19 patients can exacerbate the GBS symptoms leading to worse outcomes (7).
Although imaging in some other reports showed that COVID-19 can affect the brain (8), but in this patient, the brain MRI did not show significant damage related to coronavirus. Moreover, in contrast to the most reported GBS cases (following coronavirus infection) in whom PCR assay was negative for coronavirus in CSF (7), the result of PCR test in the CSF of our patient was positive and it prompted an ongoing controversy whether neurological symptoms are caused by a viral infection of the CNS or via other mechanisms. It was reported that coronavirus cause an excessive immune reaction with an increased level of cytokines and stimulate the inflammatory cascade leading to extensive tissue damage and these immunological processes are responsible for the major part of the organ manifestations, including the neurological complications (3, 4, 9, 10). In addition, Scheidl et al. reported that more serious respiratory symptoms in the acute phase of COVID-19 are associated with a more severe form of GBS (7). It was postulated that; COVID-19 induces the production of antibodies against specific gangliosides that may cause to appear the certain forms of GBS (10). However, as Chervet et al. reported, exchanging plasma by removing autoantibodies, immune complexes, and cytotoxic constituents from serum, can decrease recovery time by 50% in GBS patients (11). Therefore, more investigations design is needed to find out the mechanisms which are involved in GBS following COVID-19. Moreover, considering the beneficial effects of plasmapheresis in these patients, it is highly recommended to import it as a part of treatment for this disease.
In brief, this is one of the few cases of COVID-19 patients with positive PCR assay for coronavirus in CSF who had the experience of respiratory system infection prior to GBS. Therefore, GBS should be considered as a neurological outcome of COVID-19. In addition, due to the plasmapheresis beneficial effects in GBS following COVID-19, along with antiviral drugs, should be considered as a part of treatment for this disease.