1. Background
Canine distemper virus (CDV) is a member of the family Paramyxoviridae (1). The virus is endemic in many areas such as India, Denmark, Finland, Brazil, and North America (2). There are some reports of CDV in dogs of Iran. A high prevalence (55.6%) of CDV has been reported among rural dogs in the north of Iran (3). In a study performed by Avizeh et al. (2007), the prevalence of CDV was 17.52% (4) Although the CD vaccine has significantly reduced the disease, CD is endemic in many parts of the world (5, 6).
The CDV inhibits immunity in vulnerable species, and its receptor is the signaling lymphocyte activation molecule 4 (SLAM 4) (5, 6). Recently, new reports have been published showing that CDV infection can facilitate the development of diseases caused by common or emerging viruses (7-11). Morbilliviruses are transmitted by aerosols and cause clinical signs such as respiratory and gastrointestinal symptoms, which are often complicated by the addition of other pathogens. In addition, one of the features of morbillivirus infection is severe, temporary suppression of the host immune system (7).
During the first 24 hours after CDV infection, the virus accumulates in macrophages, B and T cells, and later, the virions go to the lymphoid tissues. Then, multiply in the reticuloendothelial organs (12, 13). The initial proliferation of the virus in lymphoid tissues leads to severe suppression of the immune system (14-16). After the viremia, CDV spreads to a number of epithelial tissues and the central nervous system (CNS) (13). Penetration of T lymphocytes and dendritic cells (DC) causes cutaneous manifestations of the disease (17). Lymphopenia occurs due to the reduction of the population of CD4+ T cells, CD8+ T cells, and CD21+ B cells, and CD4+ T cells are the main targets of CDV (18). CD4+ lymphocytes will be probably apoptotic and die (16), while CD8+ cells are more resistant (17).
The decrease in circulating immune system cells may be due to the reduction of cells in the lymphatic organs as well as the apoptosis of white blood cells. Programmed cell death can also happen in non-infected cells, indicating an additional non-viral mechanism of apoptosis (19). Candida albicans (C. albicans) is one of the microflora of the body, which in some cases causes intense mucosal and skin infection, vaginal candidiasis, oral candidiasis, and other fungal diseases (20). People with immunosuppression are particularly vulnerable to infections like candidiasis (21). Candida species in growth medium are in the form of little yeast cells (3 - 6 microns), egg-shaped with thin shells, and Blastoconidium. In the growth time, buds do not separate from the mother cell and create a pseudohypha (18).
The attack of C. albicans on the skin happens through impact and creating laceration or burns, and when the skin moisture is high, the spots form. Onychomycosis is one of its other consequences (22). Thrush and oral candidiasis involve the oral cavity, tongue, gums, or oral palate, which may lead to bleeding (22). The attack of yeasts to vaginal mucus results in vulvovaginitis and causes itch, inflammation, and an increase in vaginal discharge (23).
2. Objectives
The aim of this study was the molecular and immunological investigation of CDV and its co-infection with C. albicans.
3. Methods
3.1. Sampling
In this cross-sectional study, the samples were taken from dogs in clinics of Isfahan (2018 - 2019). The age was determined based on the statements of the animal owner and the examination of the animal's teeth. For sampling, the animal was first physically restrained and examined for clinical signs. After the restrain of the animals [ketamine (Alfasan, Netherlands)-acepromazine (Hogestrati, Belgium)], respiratory and gastrointestinal tract swabs were taken from 50 symptomatic and 50 asymptomatic unvaccinated dogs for examination by a rapid immunochromatography detection kit of distemper and placed in sterile PBS. One of the limitations of the present study was the achievement of a large number of symptomatic CDV cases. For this reason, a statistically appropriate sample size was not obtained.
3.2. Rapid Distemper Immunochromatography
Rapid immunochromatography kit of distemper; VetALL SensPERT Canine Distemper Virus Test Kit, CDV Ag (Ngaio Diagnostics, New Zealand) was applied for detection of CDV in eye-respiratory swabs. The samples were firstly diluted in the kit buffer. Then 100 µL of it was poured into the sample well, and from this moment, the time was taken. Then, the sample was dripped on a pad, and after the adsorption of dripped samples, two red bands showing the positive result appeared in the C (control) and T (test) positions.
3.3. Culture Test, Giemsa Staining
Culture tests were conducted on Sabouraud dextrose agar plate. After the smears were prepared on the slides, they were fixed with methanol and dried completely (30 seconds). Consequently, slides were immersed in a freshly prepared 5% Giemsa stain solution for 15 - 30 minutes. The slides were washed and dried with water and finally observed with a Bright Field microscope.
3.4. RNA/DNA Extraction and RT Reaction
QIAamp Viral RNA Mini Kit (Qiagen, Cat No: 52904) was used for RNA extraction from buffy coat. In order to determine the quality of the extracted RNA samples, the optical density (OD) of the samples was measured with a spectrophotometer at 260/280 nm. TaqMan RT Kit was used for cDNA synthesis (Invitrogen, USA) for applying in PCR reaction. The reaction was performed in a total volume of 25 µL using recommended concentration of RT buffer, MgCl2, dNTPs, Random hexamers, RNase inhibitors, and reverse transcriptase (MMLV RT). the RNA template (400 - 500 ng/μL) and distilled sterile water up to 10 μL. The thermal program included: 25°C for 10 min, 48°C for 30 min, and 95°C for 5 min. For investigation of C. albicans, DNA was extracted using the protocol suggested by Doyle et al. (1990) (cited in Davies et al.) (12).
3.5. Statistical Analysis
RT-PCR and PCR assays were performed with primers designed for detection of CDV by Beacon designer software. The sequences of specific primers of C. albicans species were extracted from Khan et al. (2001) (23). The sequences of primers were as follows: CDVF: AAGCCTCACACTGTTCAAG-3', CDVR: GATTAGGACTATAATGACATGC-3' and C.albF: ATGGGTGGTCAACATACTTC-3', C.albR: 5'-TACATCTATGTCTACCACC-3'. For detection of C. albicans, the PCR thermal cycle programs consisted of 94°C for 5 min, 30 cycles at 94°C for 45s, 60°C for 45 s and 72°C for 45 s, and 72°C for 5 min. The PCR cycling and temperature for investigation of CDV were the same as C. albicans. The only difference was that the annealing temperature was 58°C. The negative and positive controls were applied in each test. The PCR products electrophoresed in 1.5% agarose with 0.01% Green viewer (Parstous, Cat No.: B111151) along with a 50-base pair ladder (Fermentas, Cat No.: SM1133). Then two positive PCR products were sent for sequencing (Bioneer, Korea). Finally, the data were analyzed using Chi-square test by SPSS-16 software.
4. Results
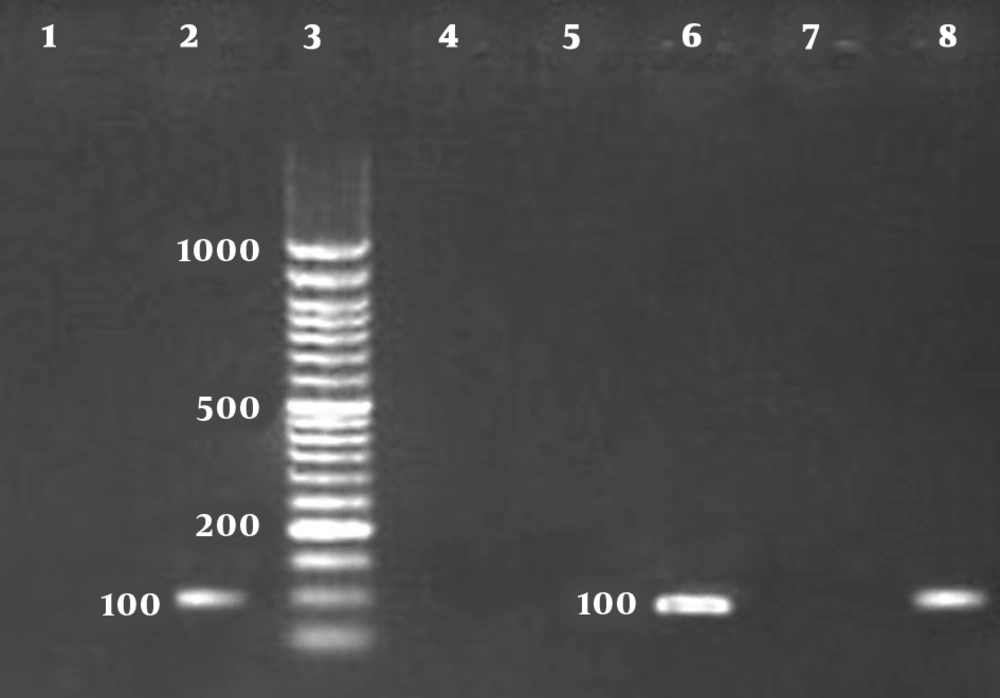
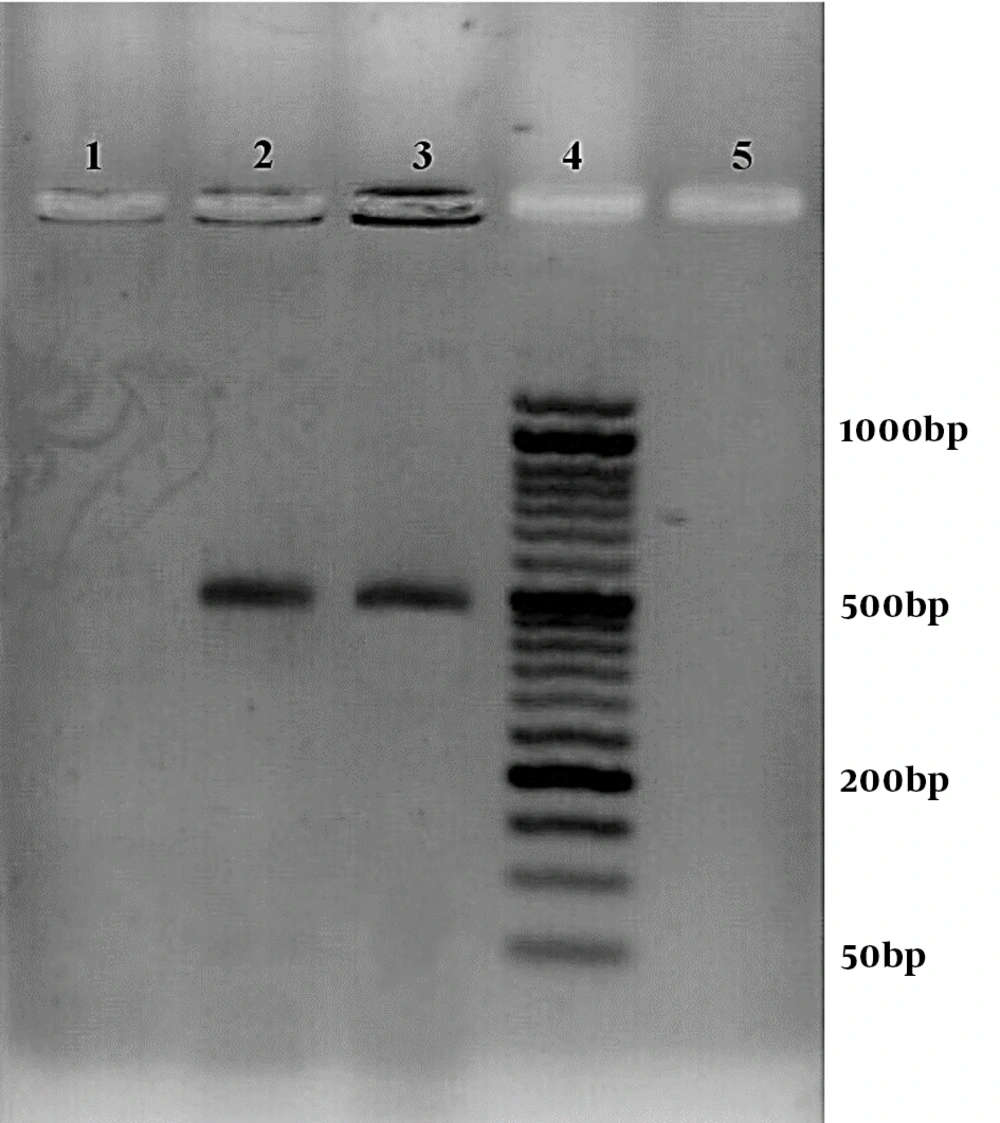
There were 29 and one positive cases among symptomatic and asymptomatic dogs using a rapid distemper immunochromatography kit. The CDV and C. albicans positive samples and controls had the bands with the sizes of 100 bp and 496 bp, respectively (Figures 1 and 2).
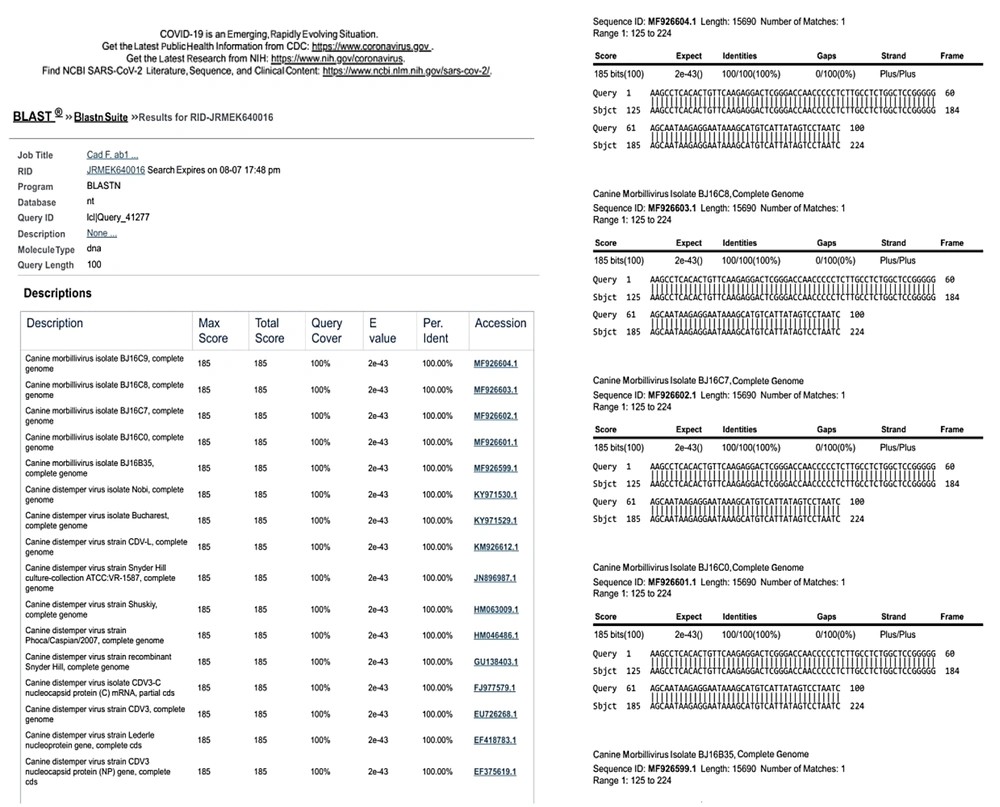
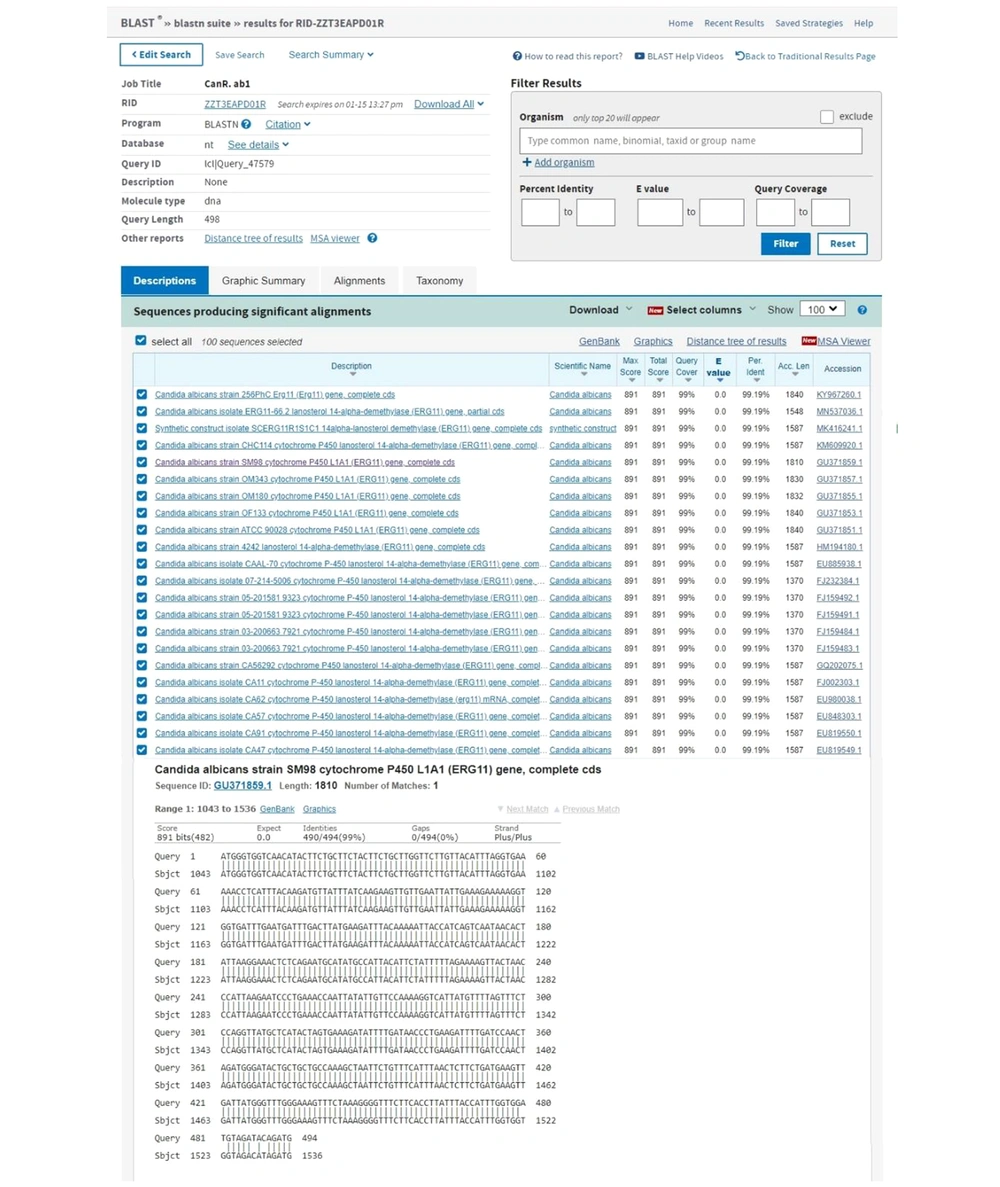
The sequencing results of RT-PCR/PCR products showed that the degree of identity of the detected sequences with the sequences registered in the gene bank for distemper virus and C. albicans were high, and it was a good indicator of PCR efficiency (Figures 3 and 4).
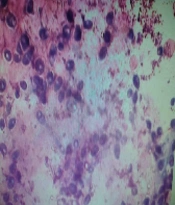
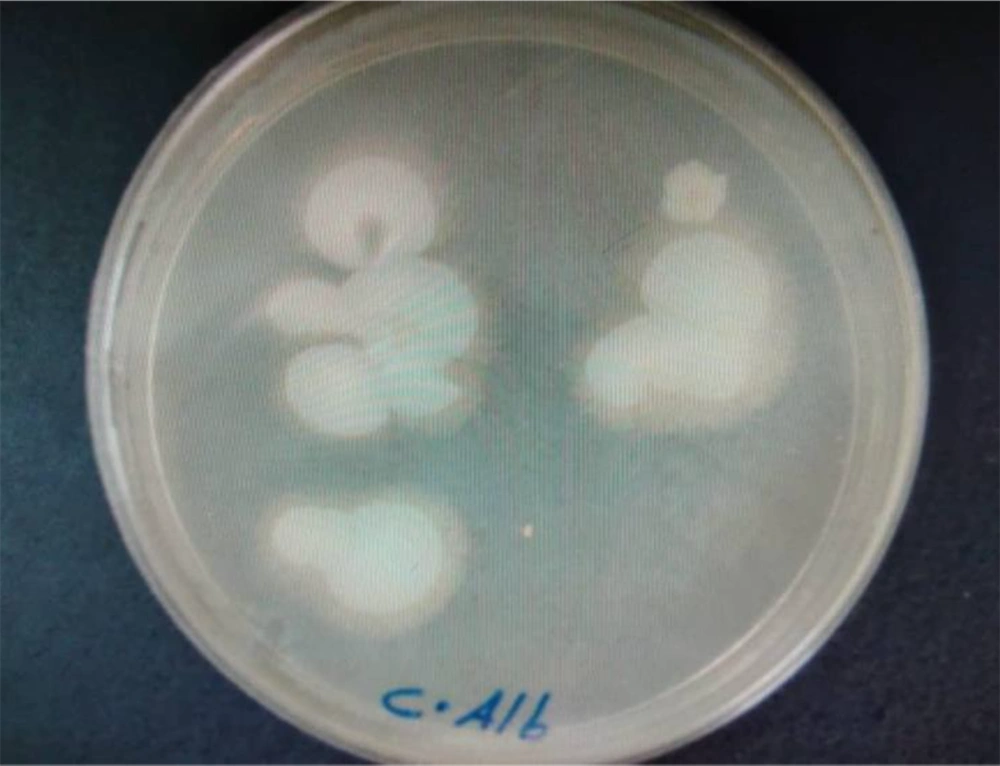
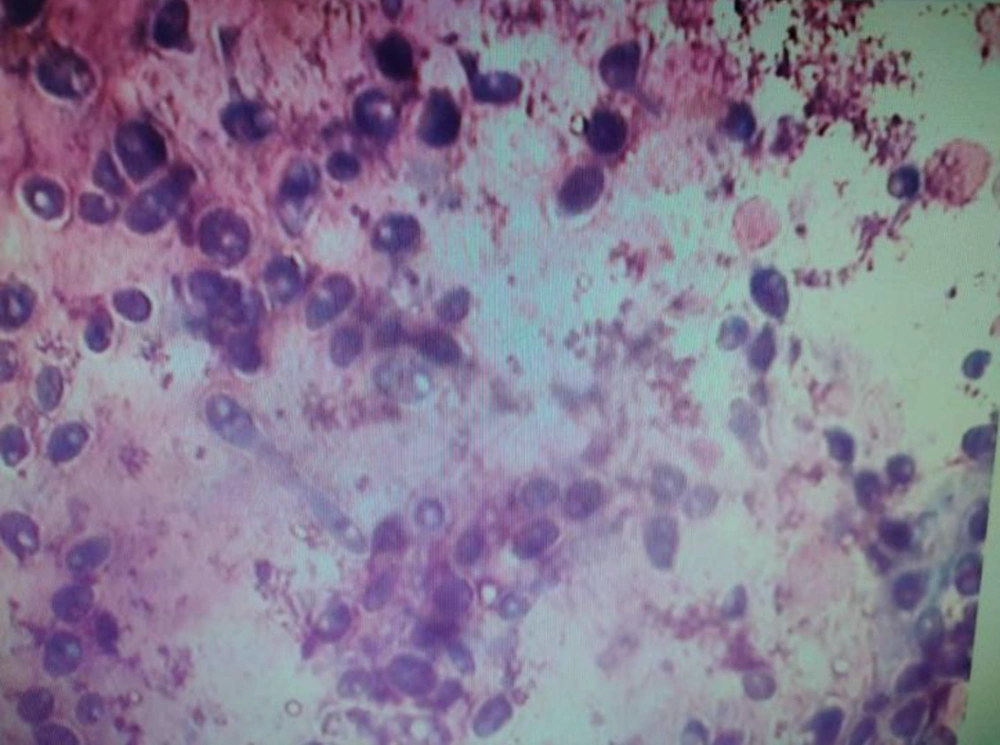
On Sabouraud dextrose agar, the smooth creamy white colonies with a characteristic yeast odor were seen. In the Giemsa stained smears, budding yeast cells and pseudomycelium were typical (Figures 5 and 6).
In total, using RT-PCR/PCR test, among 50 symptomatic and 50 asymptomatic dogs, there were 37 and three CDV-positive results, respectively. The frequency of C. albicans samples in the first and second groups were four and one cases, respectively. The rate of CDV and C. albicans coinfection was 4%, only among dogs suspected of having distemper clinical signs. All of the 30 samples were positive using a rapid distemper immunochromatography test showed positive results by RT-PCR performed for detection of CDV. There was a statistically significant relationship between the results of these two tests (P < 0.05). Furthermore, there was a statistically significant association between CDV rapid test results and possess the symptoms of distemper. No statistically significant relationship was found between two diseases, C. albicans PCR results and gender; and CDV RT-PCR results and gender (P > 0.05). However, the relationship between C.albicans PCR tests and CDV RT-PCR results and CDV signs and age groups was statistically significant (P < 0.05) (Table 1).
| Variables | CDV Rapid Test | CDV Sign | Candida albicans PCR | Gender | Age Group | CDV RT-PCR |
|---|---|---|---|---|---|---|
| CDV rapid test | - | Significant | Significant | Not significant | Significant | Significant |
| CDV Sign | Significant | - | Significant | Not significant | Not significant | Significant |
| C. albicans PCR | Significant | Significant | - | Not significant | Significant | Not significant |
| Gender | Not significant | Not significant | Not significant | - | - | Not significan |
| Age group | Significant | Not significant | Significant | - | - | Significant |
| CDV RT-PCR | Significant | Significant | Not significant | Not significant | Significant | - |
aP-Values of less than 0.05 were considered significant
5. Discussion
Almost all dogs with distemper experience immune suppression. As a result, other infections can be added to the disease and make these dogs sicker (3). Therefore, immune-suppressing mechanisms may affect the cells that are not directly involved with viral infection. Although the exact mechanism of immunosuppression due to distemper virus infection is not well understood, the weakening of the immune system due to this virus is accepted by all scientists. Therefore, and considering the possibility of further infections due to immunosuppression resulting from distemper virus, the current study was done to investigate co-infection of distemper and C. albicans as an opportunistic pathogen. However, the co-infection rate was 4% of cases, there was no statistically considerable relationship between distemper and C. albicans. In this study, the overall prevalence of C. albicans was 5%.
The limitation of the present study in accessing a higher number of symptomatic dogs and, consequently, low sample size may be one of the reasons for not achieving a considerable association between these two pathogens. The use of a rapid distemper diagnostic kit is the best option for diagnosing the virus in veterinary clinics. Its specificity and sensitivity for conjunctival specimens are similar to nested-PCR (24). There is no interference between vaccination and this diagnostic method (25). So, this assay was used for the initial diagnosis. Previously, some research groups have worked on the issue of CDV co-infection with other pathogens, some of which are mentioned below:
We could not find any report of simultaneous infections with distemper in Iran; there are some reports of CDV infection from some cities, such as a study in which serologic detection of CDV in unvaccinated dogs from Ahvaz were performed by Avizeh et al. in 2007. Furthermore, Namroudi et al. (2015) detected the Arctic and European cluster of CDV in the north and center of Iran (3, 4). In 2008, Gabriel et al. reported simultaneous infections of canine adenoviruses type-2 (CAV-2), CDV and different types of candidas in two 3-month-old dogs (5). In 2015, Headley et al. reported a new pneumonia case in a dog caused by talaromyces marneffei along with distemper infection (26). In another work, in 2015, Kubiski et al. reported co-infections of CDV and Sarcocystis species (27).
In 2006, Panasiti et al. reported a patient infected by simultaneous infections of Herpes simplex virus type-2 (HSV-2), human immunodeficiency virus (HIV), and C. albicans (28). Toplu et al. (2009) reported the co-infection of CDV, hepatozoonosis, and toxoplasmosis in 6 dogs with visceral leishmaniosis (29). Furthermore, Lemberger et al. (2005) reported the simultaneous infections of distemper virus and Neospora caninum in a dog (30).
Mochizuki et al. (2008) studied the pathogens of upper respiratory tract infections in domestic dogs. Canine parainfluenza virus (4.7%), canine coronavirus group 1 (4.4%), canine adenovirus type 2 (2.9%), canine coronavirus group 2 (1.5%), and canine distemper virus (1.5%), were detected and two cases of co-infections were reported in this study (11).
In the research performed by Damián et al. (2005), CDV was detected in 77%, CAV in 57%, and CpiV in 51% of cases, respectively. The most common co-infection was CDV-CpiV in 14% of cases (21). In the study of Headley et al. (2018), CDV-associated infections were reported as follows: N. caninum (100%), CPV-2 (100%), CAdV-1 (100%), and CAdV-2 (100%) (11). Aguiar et al. (2012) investigated the co-infection of CDV and T. gondii in dogs with neurological signs. Accordingly, 80.9% of dogs were positive for CDV using RT-PCR and 38.1% were positive for anti-T. gondii antibodies. Moreover, 41.1% were positive for both factors (6). In 2015, Headley et al. detected the co-infection of distemper virus with canine herpesvirus type 1, adenovirus and parvovirus in domestic dogs in southern Brazil (6).
The results of all mentioned researches indicate the high rate of co-infection of distemper with other pathogens confirms the hypothesis of increasing the susceptibility to other pathogens due to CDV immunosuppression. However, performing more similar studies on other hosts of the virus can give us complete information about this issue.
5.1. Conclusions
In total, the frequency of co-infection was four cases among the samples taken from symptomatic dogs and any statistically considerable association was not observed between distemper and C.albicans.