1. Background
Liver fibrosis as a metabolic disorder is currently a global problem. The transplantation of all or part of the liver tissue is the only solution to treat patients with acute liver fibrosis; therefore, it is important to find other treatments. Hepatic stellate cells (HSCs) are the main mediators of liver fibrosis. When HSCs activate, they produce the extracellular matrix (ECM) and collagen, type I, alpha 1 (collagen1α) in response to damage to hepatocytes (1, 2). The damage can be caused by various factors, including severe viral hepatitis and non-alcoholic steatohepatitis (NASH) (3). If left untreated, the excessive accumulation of the ECM proteins ultimately leads to liver failure and hepatocellular carcinoma (4).
Among the factors that can increase the activity of star-shaped cells, it can be said that the transforming growth factor-beta (TGF-β) plays the most important role. According to previous studies on liver fibrosis pathways, the increased activation of the TGF-β signaling pathway could eventually lead to the production of more proteins, such as collagen1α, thereby accumulating the ECM (5). The primary activation of the TGF-β signaling pathway is through well-known receptors called serine/threonine kinase at the cell surface (6). In some studies, it has been suggested that by inhibiting the activation of the TGF-β and its downstream pathways, it is possible to prevent the activation of as many star-shaped cells as possible. Therefore, by inhibiting the effects of TGF-β, fibrogenic conditions in the liver can be improved (7, 8).
The activation of toll-like receptor 4 (TLR4) signaling leads to the downregulation of the transmembrane inhibitory TGF-β pseudoreceptor BMP and activin membrane-bound inhibitor (BAMBI) on HSCs, a critical fibrogenic cell type in the liver. As a result, net TGF-β1 activity increases, enhancing the ECM production (9, 10). Fibroblast growth factor 21 (FGF21) is a natural secretory protein in the body with effects, such as the reduction of fat accumulation and oxidation of lipids (11). Animal experiments showed that the administration of recombinant FGF21 has beneficial effects on fatty liver and hepatic steatosis and improves fibrotic conditions and liver toxicity (12). However, no study has investigated the ability of FGF21 to protect against hepatic fibrosis in cholesterol-treated human HSCs.
2. Objectives
The present study aimed to investigate the effect of FGF21 to reduce cholesterol-treated activated human HSCs. The obtained results demonstrated that FGF21 inhibited TLR4 signaling, thereby inhibiting the activation of HSCs; therefore, FGF21 can act as an influential factor in preventing liver fibrosis.
3. Methods
3.1. Materials
Cell culture grade cholesterol and FGF21 were provided from Sigma-Aldrich (USA) and Abnova (Taiwan), respectively. Fetal bovine serum (FBS) from Gibco (USA), Dulbecco’s modified Eagle’s Medium (DMEM) Low Glucose, and Pen/Strep from Bio-Idea (Iran) were purchased. Next, FGF21 (final concentration of 1.0 μM) was dissolved in a culture medium and added to the cells for 24 h. The present study was designed and conducted with the permission of the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (IR.AJUMS.REC.1399.649).
3.2. Cell Culture
The LX-2 cell line (i.e., an immortalized human HSC line) as an HSC cell line was kindly provided by Scott L. Friedman (Mount Sinai School of Medicine, USA). The cells were seeded in DMEM containing 10% FBS and penicillin and streptomycin antibiotics (100 mg/mL each) in a 5% CO2 humidified incubator at 37°C.
3.3. Treatment of LX-2 Cells with Different Cholesterol Concentrations
For cholesterol treatment, the cells were plated in 6-well plates at the density of 105 cells per well and grown in the 5% CO2 incubator at 37°C until 80% confluency. The cells were serum-starved for 24 h before treatments and then treated with different concentrations of cholesterol, including 25, 50, 75, 100, 125, and 150 μM, for 24 h; then, the cells were subject to serum starvation for 24 h.
3.4. Treatment of Cholesterol-Activated HSCs with FGF21
In the following step, for the inspection of the inhibitory effects of FGF21 on activated HSCs, cholesterol-activated HSCs (75 and 100 μM) were treated by FGF21 (1 μM) for 24 h. Real-time polymerase chain reaction (PCR) was performed to evaluate the messenger ribonucleic acid (mRNA) expression of genes involved in liver fibrogenesis.
3.5. MTT Assay Technique
MTT (3-(4,5-Dimethylthiazol 2-yl)-2,5-diphenyltetrazolium bromide) assay was performed to evaluate cell survival in the presence of cholesterol compound. In each well of 96 cells, about 5000 cells were cultured and incubated for 24 h. Then, the cells of each well (13) were treated with various cholesterol concentrations of 25, 50, 75, 100, 125, and 150 μM for 24 h in an incubator at 37°C and 5% CO2. Then, the medium was changed, and the MTT compound (0.5 mg/mL) was added to the cells and incubated for 4 h. The MTT-containing medium was then removed, and 100 μL of dimethyl sulfoxide was added to each well to dissolve formazan crystals. Finally, the absorbance of the samples at 595 nm was determined.
3.6. Quantitative Real-time PCR
Ribonucleic acid (RNA) was extracted by a kit of Qiagen Company (Germany) according to the manufacturer’s instructions. The optical density wavelength of 260 to 280 readings and the number 2.0 were obtained using the nanodrop device. Complementary deoxyribonucleic acid synthesis was then performed on total RNA. For the quantification of the mRNA expression, real-time PCR was performed using RealQ Plus 2x Master Mix Green “Low Rox” kit (Ampliqon, Denmark) and QuantStudioTM 3 Real-time PCR System (ABI Applied Biosystems, USA) consisted of a hot start by heating the PCR solution to 95°C for 10 min, followed by 38 cycles of denaturation at 95°C for 10 sec and annealing/extension at 58°C for 1 min. The total time of running this protocol on the system of the current study was 90 min with the following specific primers:
TGF-β: forward 5'-GTGGACATCAACGGGTTCACT-3', reverse: 5'- CTCCGTGGAGCTGAAGCAATA-3';
TLR4: forward 5'- GCCAGGATGATGTCTGCCTC-3’, reverse 5'-TTAGGAACCACCTCCACGC-3';
BAMBI: forward 5'- GACAGACATCTGCCAAGCCA-3’, reverse 5'-TGATACCTGTTTCCTTGTCCTGA-3';
Collagen1α: forward 5'-GGAATGAAGGGACACAGAGGTT-3’, reverse 5'-AGTAGCACCATCATTTCCACGA-3';
Glyceraldehyde 3-phosphate dehydrogenase: forward 5'-GACAGTCAGCCGCATCTTCT-3', reverse 5'-GCCCAATACGACCAAATCCGT-3'
3.7. Statistical Analysis
All the experiments were performed in triplicate. The data were analyzed as mean ± standard error of the mean using analysis of variance and Tukey’s posthoc test using GraphPad Prism software (version 9.0.1). The significance level was considered less than 0.05.
4. Results
4.1. Effect of Different Cholesterol Concentrations on the Survival of HSCs
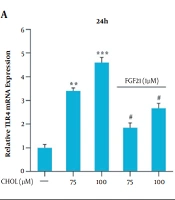
The MTT assay was performed to achieve appropriate concentrations of cholesterol for cell treatment. For this purpose, the cells were first treated with various cholesterol concentrations of 25, 50, 75, 100, 125, and 150 μM for 24 h. The control group was not treated with cholesterol. At a concentration of 125 μM cholesterol, the percentage of star-shaped cells was significantly reduced, compared to that of the control group (P < 0.001; Figure 1). Therefore, concentrations lower than the half-maximal inhibitory concentration were used for the experiment.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay results showing cell viability under different cholesterol concentrations over 24 h. Results are shown as mean ± standard error of the mean. Statistical analyses were performed using GraphPad Prism software (version 9.0.1), one-way analysis of variance, and Tukey’s test. Statistical significance was considered < 0.05 (** P < 0.01 and *** P < 0.001).
4.2. mRNA Expression of TLR4 and BAMBI Genes in the Presence of Cholesterol and FGF21
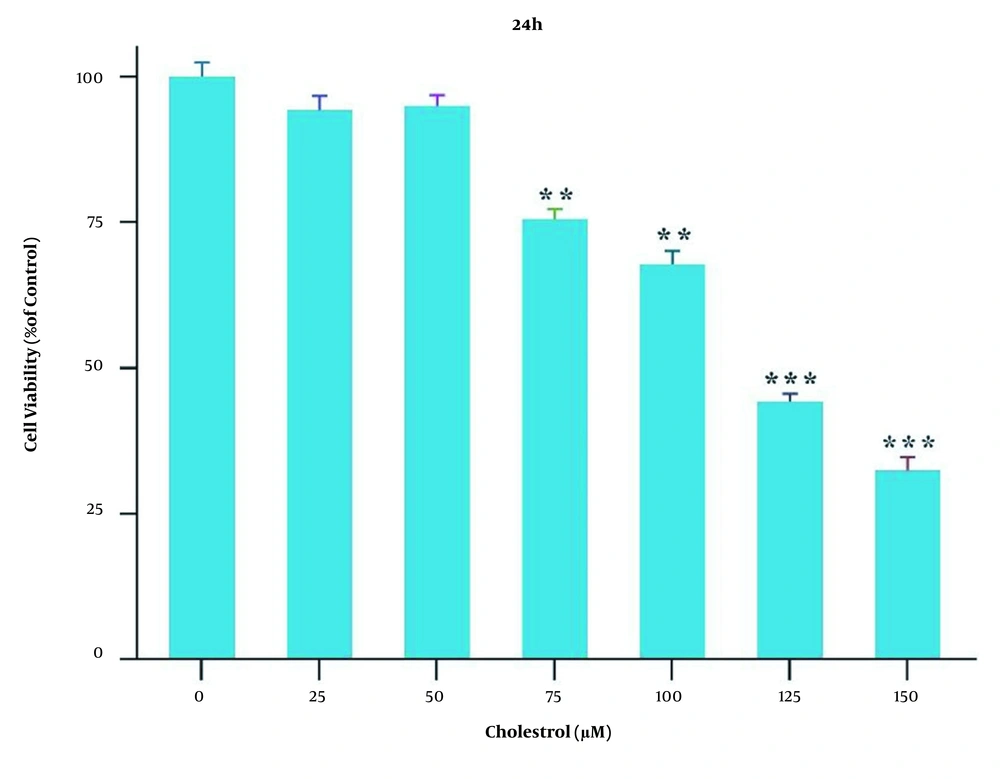
Qualitative real-time PCR was used for the evaluation of the mRNA expression of TLR4 and BAMBI genes in the presence of different cholesterol concentrations. The results showed that the mRNA expression of these genes did not significantly change in the presence of 25 and 50 μM cholesterol; however, in the presence of 75 and 100 μM concentrations, the mRNA expression of the TLR4 gene significantly increased, and the mRNA expression of the BAMBI gene significantly reduced, compared to those reported for the control group (* P < 0.05, ** P < 0.01, and *** P < 0.001; Figure 2A). The modulation of TLR4 and BAMBI genes by 1 μM FGF21 was evaluated following their induction with 75 and 100 μM cholesterol. Qualitative real-time PCR results showed that the mRNA expression of the TLR4 gene significantly reduced and the BAMBI gene significantly increased in response to FGF21, compared to those reported for the cells treated with 75 and 100 μM cholesterol (# P < 0.05; Figure 2B).
Toll-like receptor 4 (TLR4) and BMP and activin membrane-bound inhibitor (BAMBI) messenger ribonucleic acid (mRNA) expression in the presence of cholesterol and fibroblast growth factor 21 (FGF21) in LX-2 cell line. A, TLR4 mRNA expression in the presence of cholesterol and FGF21 in LX-2 cells. B, BAMBI mRNA expression in the presence of cholesterol and FGF21 in LX-2 cells. The results of three replications (mean ± standard error of the mean) and changes in TLR4 and BAMBI mRNA expression were compared to those of the control group. The significance level was considered < 0.05. Glyceraldehyde 3-phosphate dehydrogenase was used as the reference gene (* P < 0.05, ** P < 0.01, and *** P < 0.001; # P < 0.05).
4.3. mRNA Expression of TGF-β and Collagen1α Genes in the Presence of Cholesterol and FGF21
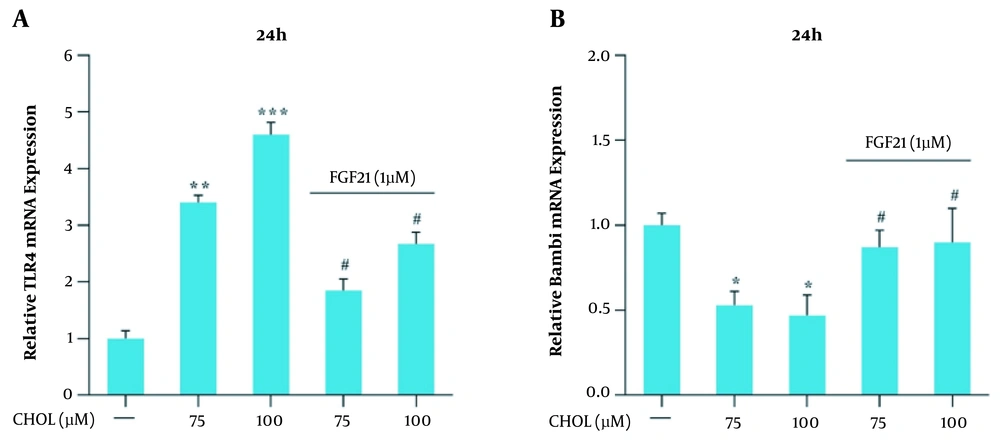
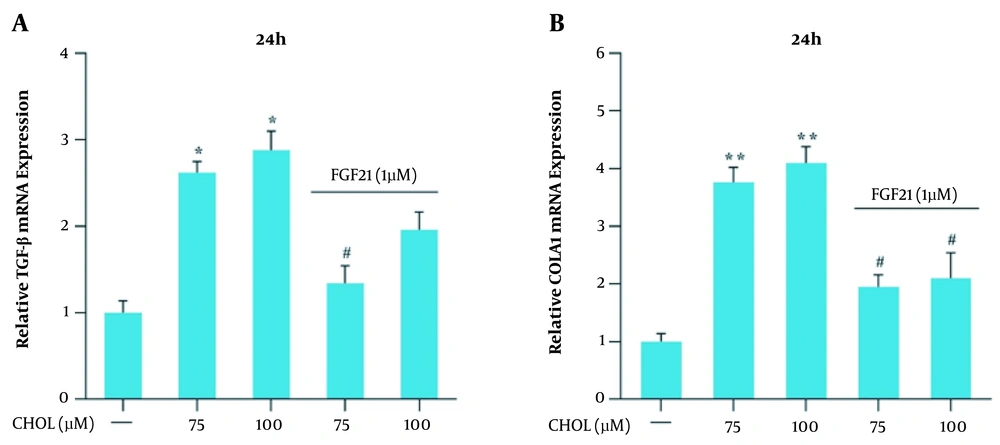
Qualitative real-time PCR was used for the evaluation of the mRNA expression of the TGF-β and collagen1α genes in the presence of different cholesterol concentrations. The obtained results showed that the mRNA expression of these genes did not significantly change in the presence of 25 and 50 μM cholesterol; nevertheless, in the presence of 75 and 100 μM cholesterol, the mRNA expression of TGF-β and collagen1α genes significantly increased, compared to that reported for the control group (* P < 0.05 and ** P < 0.01; Figure 3A). The modulation of TGF-β and collagen1α genes expression by 1 μM FGF21 was evaluated following their induction with 75 and 100 μM cholesterol. Real-time PCR results showed that the mRNA expression of TGF-β and collagen1α genes significantly reduced in response to FGF21, compared to that reported for the cells treated with 75 and 100 μM cholesterol (# P < 0.05; Figure 3B).
Transforming growth factor-beta (TGF-β) and collagen, type I, alpha 1 (collagen1α) messenger ribonucleic acid (mRNA) expression in the presence of cholesterol and fibroblast growth factor 21 (FGF21) in LX-2 cell line. A, TGF-β mRNA expression in the presence of cholesterol and FGF21 in LX-2 cells. B, collagen1α mRNA expression in the presence of cholesterol and FGF21 in LX-2 cells. The results of three replications (mean ± standard error of the mean) and changes in TGF-β and collagen1α mRNA expression were compared to those of the control group. The significance level was considered < 0.05. Glyceraldehyde 3-phosphate dehydrogenase was used as the reference gene (* P < 0.05 and ** P < 0.01; # P < 0.05).
5. Discussion
Hepatic fibrosis is a significant health problem worldwide for which there is no acceptable therapy. The most important reason for hepatic fibrosis is the excess deposition of collagen1α. These activated HSCs secrete the ECM in hepatic fibrogenesis. Therefore, collagen1α is a helpful marker for the assessment of hepatic fibrosis (14). Several studies have been performed to understand the molecular mechanisms that lead to the expansion of hepatic fibrosis (15).
It has been previously reported that TLR4 enhances TGF-β signaling in HSCs, HSC activation, and hepatic fibrosis (16, 17). Recognizing the factors involved in the onset and progression of liver fibrosis are important for the prevention of this disease. The HSCs are central contributors to the initiation and progression of liver fibrosis (18). Therefore, it is important to determine the factors that cause HSCs activation and develop hepatic fibrosis.
In this study, the effects of several cholesterol concentrations were studied on the activation of LX-2 cells and changes in the mRNA expression of fibrogenic genes. The present study showed that at high concentrations, cholesterol could activate human HSCs and increase the mRNA expression of fibrogenic genes; nevertheless, no such changes were observed at lower concentrations.
To date, numerous efforts have been made to find effective drugs to prevent the progression of liver fibrosis; however, it is still a great problem to find drugs that can control the progression of liver fibrosis. The FGF21 is a natural secretory protein in the body with effects, such as the reduction of fat accumulation and oxidation of lipids. Recently, the administration of FGF21 has been shown to have several metabolic benefits on insulin sensitivity and reduce the accumulation of fat in the liver in obese rats without specific side effects (19, 20).
Studies on humans have shown high levels of circulating FGF21 in patients with type 2 diabetes and non-alcoholic fatty liver disease/NASH (21). On the other hand, previous studies showed that the administration of recombinant FGF21 has beneficial effects on fatty liver and hepatic steatosis and improves fibrotic conditions and liver toxicity (22, 23). The current study investigated whether FGF21 can reduce activated human HSCs treated with cholesterol. The result of the present study showed that FGF21 reduced activated human HSCs treated with cholesterol. In this study, FGF21 significantly reduced the mRNA expression of TGF-β, collagen1α, and TLR4 genes in cholesterol-treated HSCs.
In summary, FGF21 can reduce the activation of LX-2 cells treated with cholesterol in vitro. The relevant mechanism is probably through the inhibition of the TLR4/TGF-β signaling pathway. However, the present study was subject to some limitations, and the results have to be observed in light of these limitations. All the experiments were carried out on cells cultured and treated in a specific cell line in a laboratory. It is advised to test on living organisms, including mice or humans. Hepatic fibrosis is a dynamic process resulting from the cooperation of HSCs with other liver cell types that warrants further examination on the effect of cholesterol and FGF21 on all hepatic cells’ interactions. It is necessary to perform further studies to evaluate the cholesterol effect on mice to measure liver damage and inflammation by measuring liver enzymes and staining liver tissue.
5.1. Conclusions
This study showed that high cholesterol treatment activates human HSCs; nevertheless, cholesterol has no such effect at lower concentrations. The FGF21 is effective in reducing the activation of HSCs treated with cholesterol in vitro. Therefore, FGF21 has significant antifibrotic properties in liver fibrosis.