1. Background
Currently, gastrointestinal cancers, especially colorectal and gastric cancers, are the major causes of malignancy. Gastrointestinal cancers are the most common cancers among men and the second most common cancers in Iranian women (1). Colorectal cancer is the third most common cause of cancer death in the world. Gastric cancer with 40% and colorectal cancer with 34.5% are the most common gastrointestinal cancers in Iran. According to statistics, gastric cancer is the first and third most common cancer in men and women in Iran, respectively (2).
Tumor prognosis plays an important role in treatment strategy. Lymph node involvement, depth of tumor invasion, and tumor markers are factors in determining the prognosis of cancers (3). These biological markers include cytokeratins. Cytokeratins are intermediate filaments composed of 20 subtypes mainly observed in epithelial cells and are among important components of the skeleton involved in stabilizing the nucleus and maintaining cell morphology (4). Currently, researchers are focusing on the relationship between cytokeratins and the type of malignancy, grade, and prognosis of tumors. Cytokeratin 7 (CK7) and Cytokeratin 20 (CK20) are the most useful cytokeratins for the diagnosis and differentiation of cancers. Simultaneous expression of these two cytokeratins has been studied in different cancers (5).
The CK7 is a type II basic low molecular weight cytokeratin, which is expressed in the epithelium of the glands and tubes of the lungs, cervix, bile ducts, ureters in the kidneys, and neurothelium; however, it is not present in squamous cell carcinoma (6).
The CK20 is observed in gastric and colon adenocarcinomas. Cytokeratins can be examined by immunohistochemical methods. The expression patterns of CK7 and CK20 in tumors are different, and these patterns have been used in the differential diagnosis of different tumors. For example, colorectal epithelial cells and their derived tumors are CK7 negative, and CK7 negative and CK20 positive patterns are used to confirm the origin of colorectal metastatic tumors (7). Nevertheless, the expression patterns of CK7 and CK20 in gastric adenocarcinoma have different frequencies.
According to reports, the most common patterns in gastric adenocarcinoma are CK7 positive and CK20 negative. The CK7 is also absent in the normal gastric mucosa; nonetheless, its expression is observed in conditions of chronic mucosal stimulation, such as Helicobacter pylori (8). Some studies have shown that the expression of CK7 in invasive breast cancer is increased, compared to that in normal breast. The early detection of gastrointestinal cancers, especially gastric and colorectal cancers in the early stages, and identification of prognostic factors play an important role in treatment strategy. One of the issues emphasized in the prognosis of gastrointestinal cancers is the use of immunohistochemistry to identify various molecules, including tumor markers (9).
2. Objectives
Therefore, the current study aimed to evaluate CK7 expression level and its relationship with some prognostic factors, such as lymph node involvement and degree of malignancy.
3. Methods
3.1. Case Selection and Tissue Samples
The present cross-sectional study employed the descriptive-analytical method. Tissue sampling was based on the archive. The pathologic records of gastric and colorectal adenocarcinomas were retrieved from the archive of the Pathology Department, Imam Khomeini Hospital, Ahvaz, Iran, within 2018 - 2019. The hematoxylin-eosin stained slides were reviewed. The samples with sufficient tissue material and complete medical record information were selected in this study. The inclusion criteria were adequate tumoral mass, absence of necrosis/hemorrhage, presence of lymph node pathologic slides, and complete medical records. The total number of gastric and colorectal adenocarcinoma paraffin samples during 2018 - 2019 was considered the sample size. Based on the inclusion criteria, 75 formalin-fixed paraffin-embedded samples were enrolled in this study. The data, including tumor size (measured in cm), tumor grade (i.e., well, moderate, and poorly differentiated), depth of tumor invasion, and lymph node status, were obtained from the files recorded at the Pathology Department.
3.2. Immunohistochemical Test
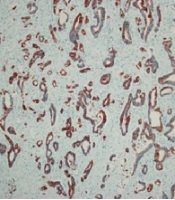
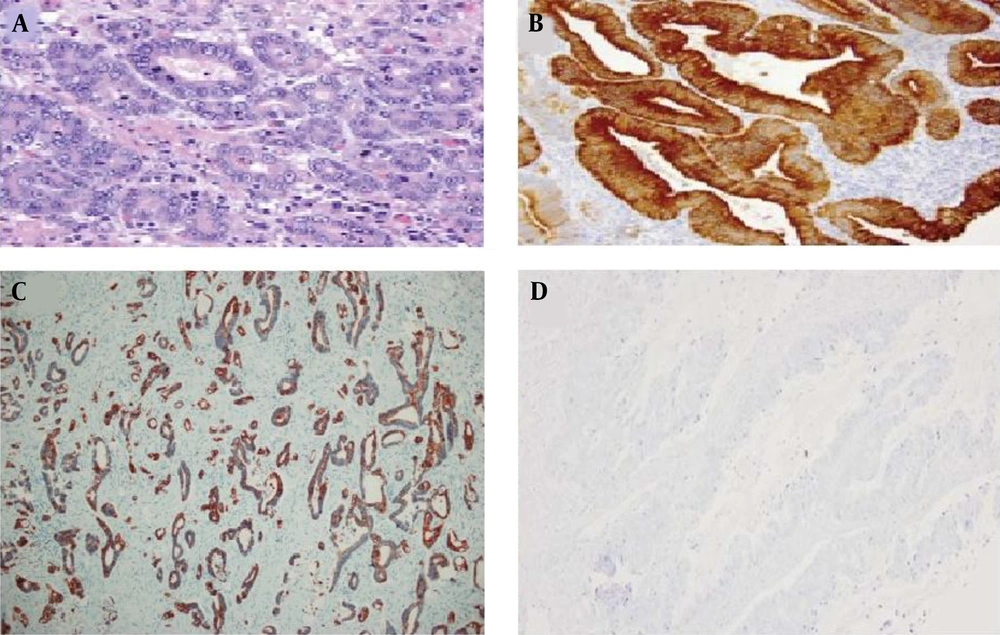
The 5-μm paraffinized sections were soaked in the water-alcohol solution for 5 min. The slides were placed in a microwave oven for 30 min at 60°C. Deparaffinization was performed by soaking the slides in xylene and then alcohol (within 75 - 100% concentration) for 5 - 10 min. The sections were rinsed with 10% phosphate-buffered saline (PBS) followed by H2O2/methanol (1: 9) and 10% PBS for 10 min. Then, the slides were heated in the microwave oven for 10 min in ethylenediaminetetraacetic acid. The samples were left to reach room temperature and then rinsed with PBS. The sections were incubated with 1 μg/mL diluted CK7 primary anti-mouse monoclonal antibody for 1 h at room temperature (DAKO, Clone OV-TL, Denmark) and then reincubated with biotinylated antibody for 30 min and soaked in 10% PBS for 10 min. The sections were incubated with conjugated enzyme for 30 min and developed in 3,3′-diaminobenzidine-tetrahydrochloride-dihydrate. Hematoxylin stain was used to develop the ground contrast. The slides were passed in distilled water-alcohol within 75 - 100% for 3 - 5 min and immersed in xylene before mounting for 5 min. The presence of CK7 was scored under high power (400x) in 1000 tumor cells, and the percentage of positive immunostaining (5%) was determined.
3.3. Statistical Analysis
For the quantitative variables, mean and standard deviation were used to describe the data center and data scatter, respectively. For the qualitative variables, frequency and percentage were used to describe the data. The Spearman’s correlation coefficient and chi-square tests were used to determine the relationship between the variables. A p-value of less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS software (version 22).
3.4. Ethical Considerations
The study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (IR.AJUMS.HGOLESTAN.REC.1399.097). Written informed consent was obtained from each patient.
4. Results
In total, in the present study, 75 gastric and colon adenocarcinomas in the pathology department were evaluated for CK7 staining. The highest frequency of the samples was related to colon cancer, with 33 cases (44%). In terms of gender distribution, there were 14 men (50%) and 14 women (50%) in gastric cancer; however, in colon-rectal cancer, there were 25 men (53.2%) and 22 women (46.8%). The frequency distribution of gender variables was similar in both groups of cancer patients (P = 0.789).
The mean age values of the patients were 62.11 ± 14.13 and 55.23 ± 14.14 years in gastric cancer and colorectal cancer groups, respectively. The mean age of the patients in the two groups of cancer patients was statistically significant (P = 0.045). According to the findings, the mean age of patients in the gastric cancer group was higher than that reported for the patients in the colorectal cancer group.
The mean tumor sizes were 4.2 ± 2.02 and 4.4 ± 2.2 cm in gastric cancer and colorectal cancer groups, respectively. There was no statistically significant difference in the mean tumor size between the two groups of cancer patients (P = 0.678). Cancer cells with a positive CK7 index were observed with brown staining of the cytoplasm. The findings of the present study showed that 19 cases (67.9%) of gastric cancer samples, 6 cases (42.9%) of rectal samples, and 14 cases (42.4%) of colon samples were positive for CK7 (Figure 1).
There was a significant relationship between cancer type and cytokeratin expression. The results showed that in gastric cancer, the expression of positive cytokeratin was higher than that of the rectum and colon, which was statistically significant (P = 0.034). The examination of the frequency distribution of differentiated gastric cancer tissue based on the degree of positive CK7 showed that regardless of the degree of tumor differentiation, the CK7 index was positive in 19 samples (70.4%) and negative in 8 samples (29.6%). In addition, there was no statistically significant relationship between the type of differentiation and cytokeratin index (P = 0.787).
The examination of the frequency distribution of differentiated colorectal cancer tissue based on the degree of CK7 positivity showed that without considering the degree of tumor differentiation, the CK7 index was positive in 19 samples (42.2%) and negative in 26 samples (57.8%). Furthermore, there was no statistically significant relationship between the type of differentiation and cytokeratin index (P = 0.949). The examination of the frequency distribution of lymph nodes in gastric cancer based on the degree of positive CK7 showed that the CK7 index was positive in 11 samples (73.3%) with lymph node involvement. However, there was no significant relationship between lymph node involvement and CK7 index (P = 0.505). The examination of the frequency distribution of lymph nodes in colorectal cancer based on the degree of positive CK7 showed that the CK7 index was positive in 9 samples (42.9%) with lymph node involvement. However, there was no significant relationship between lymph node involvement and CK7 index (P = 0.605).
The examination of the frequency distribution of perineural invasion in gastric cancer based on the degree of CK7 positive showed that the CK7 index was positive in 13 samples (76.5%) with perineural involvement. However, there was no significant relationship between perineural invasion and CK7 index (P = 0.225). The examination of the frequency distribution of perineural invasion in colorectal cancer based on the degree of CK7 positivity showed that the CK7 index was positive in 10 samples (50%) with perineural involvement. However, there was no significant relationship between perineural invasion and CK7 index (P = 0.374).
5. Discussion
The present study was performed on 75 samples of gastric and colorectal adenocarcinoma blocks. The results showed that most CK7 gastric adenocarcinomas were positive; nevertheless, most CK7 colorectal adenocarcinomas were negative. Moreover, the results of this study showed that there was no statistically significant relationship between prognostic factors (i.e., lymph node involvement, neuronal invasion, and type of tumor differentiation) in both types of cancer.
Previous studies have shown that the expression of CK7 and CK20 can be effective in gastric and colorectal cancers in the diagnosis of pathological tumor metastasis. Furthermore, different expression patterns of CK7 and CK20 in tumors of epithelial origin are useful tools in the differential diagnosis of carcinoma (7). The CK7 is the cytoplasmic filament of epithelial cells. According to the specific CK profile of cancer, there is a possibility to find the relationship between Ck7 expression and other prognostic factors (10). Most efforts to express CK7 and CK20 are focused on different metastatic carcinomas to identify their source. However, available data on the association of CK expression patterns with clinicopathological parameters are rare (11).
According to reports, the most common expression patterns of CK7 and CK20 in colorectal adenocarcinoma are CK20 positive and CK7 negative; nonetheless, the most common expression patterns of CK7 and CK20 in gastric adenocarcinoma are CK20 negative and CK7 positive (12). Therefore, it can be concluded that the expression patterns of CK7 in gastric and colorectal carcinomas are different and contradictory. In various studies, the expression level of CK7 has been reported within the range of 10 - 75%, which can be due to differences in sample size, grouping, and kit sensitivity. Prognostic factors are used to determine the best treatment management. Previous studies have shown CK7 expression in several colon and gastric adenocarcinomas (10).
In the present study, it was shown that the incidence of CK7 in gastric cancer was higher in gastric carcinoma with moderate and poor differentiation; however, in colorectal cancer, also the incidence of CK7 was higher in carcinomas with moderate and well differentiation. In general, in the present study, there was no significant relationship between the CK7 expression and degree of tumor differentiation in gastric and colorectal cancers. Park et al. showed that there was no significant relationship between the incidence of CK7 and CK20 and degree of tumor differentiation (7). Bayrak et al. showed that CK7 expression was higher in tumors with lymph node involvement; nonetheless, CK20 positive was higher in low-grade tumors (11).
Driessen et al. examined the combined immunohistochemical expression of CK7 and CK20 in 214 resection formalin-fixed paraffin-embedded specimens for adenocarcinoma, including 66 esophageal, 73 cardiac, and 75 distal gastric adenocarcinomas. Their results showed that CK7+/CK20− expression pattern was the most common pattern in tumors situated at the gastroesophageal junction, esophageal, and cardiac adenocarcinomas (67 vs. 68%); however, this cytokeratin pattern was uncommon in distal gastric adenocarcinomas (13).
Jovanovic et al. showed that CK7 was not observed in the normal mucosa of the large intestine, although it was observed in a few cases with inflamed and damaged mucosa. This marker is observed in all dysplastic cells and has nothing to do with the severity of dysplasia. Additionally, some adenocarcinomas were CK7 positive. Therefore, the colonic dysplasia lesions of the large intestine appear to be CK7 positive (14).
Gheini et al. (1) reported a statistically inverse relationship between the incidence of CK20 and CK7 with the degree of tissue differentiation and involvement of lymph nodes in gastric cancer. Although there was a statistically significant relationship between the incidence of tissue invasion in gastric cancer and incidence of CK7, no association was observed between the incidence of CK20 and tissue invasion. Gheini et al. (1) concluded that a decrease in the expression of CK20 and CK7 was associated with a decrease in tissue differentiation and an increase in lymph node involvement. Previous studies have shown that CK7 expression is of little value in the differential diagnosis of metastatic carcinoma; nevertheless, in some tumors, such as prostate and hepatocellular carcinomas, the absence of cytokeratin is of diagnostic value (15).
In the present study, no association was observed between lymph node involvement and CK7 expression. Bayrak et al., in a study conducted on 196 samples with colorectal adenocarcinoma, demonstrated a significant association between CK7 positivity and lymph node involvement. This difference may be due to the sample size (16).
The profile analysis of CK7 and CK20 is one of the methods used to determine the origin of ovarian tumors. If CK7 is positive, it is most likely an ovarian tumor. Some ovarian metastases from adenocarcinoma of the gastric and colon and an increase in the size of these tumors lead to an increase in CK7 expression. Therefore, CK7 expression cannot rule out metastasis in gastrointestinal adenocarcinoma (10). Additionally, Kummar et al. obtained similar results. They evaluated the negative expression of CK7 in the diagnosis of metastatic lung tumors of the colon. Based on the results, the expression of CK7 could be a sign of lung origin. In the aforementioned study, out of 24 metastatic cases, 2 cases were positive for CK7. Again, given the lack of a large number of these cases, caution should be exercised in dealing with the aforementioned results (17).
Park et al. showed that all of the 225 cases of metastatic colorectal carcinomas to the ovary were CK7-/CK20+, and only one case was CK7-/CK20-; accordingly, 75% of low-grade and 52% of high-grade carcinomas were CK7-/CK20+ (7). Gheini et al. demonstrated that CK7-/CK20+ was the most prevalent pattern in colon adenocarcinoma. Furthermore, they reported an insignificant difference in histopathologic grade (P = 0.26), lymph node involvement (P = 0.46), and depth of invasion (P = 0.22) in different CK7/CK20 expression patterns (18).
Differences in sample size, type of used antibody clone, and type of tumor subtype are among the reasons for the variations in the results of different studies conducted on CK7 expression. The lack of access to complete clinical staging and reduction of the number of samples to comply with the inclusion criteria were the limitations of the present study. Due to the findings of the present study and the small number of performed studies, it is required to perform further studies with a larger sample size and longer follow-up to evaluate the exact effect of this factor on the prognosis.
5.1. Conclusion
The present study showed no association between CK7 expression and prognostic factors in colon and gastric adenocarcinomas. Given these findings and several studies in this field, it is required to perform further studies with a larger sample size to determine the exact prognostic role of this factor.