1. Background
Ovarian malignancy is one of the most common causes of death concerning female reproductive system cancers (1, 2). Patients undergo surgery and receive chemotherapy with a drug containing platinum (3). Today, the use of safer alternatives, such as herbal remedies for cancer, has increased. Ginkgo biloba is a herbal medicine showing anti-cancer effects (4, 5). Effective compounds of this plant include flavonoid glycosides and their antioxidant properties (6). The administration of drugs that have previously been used to treat noncancerous diseases is another strategy to overcome the side effects of chemotherapy, pharmacodynamic, pharmacokinetic, and toxicity properties of which are well known (7).
Flunixin can act as a nonselective inhibitor of cyclooxygenases because it is a chemical compound, anti-inflammatory, and nonsteroidal. Flunixin is also used as an analgesic and antipyretic drug in medicine and exerts its inflammation effect by inhibiting the production of prostaglandins. The effect of flunixin has been confirmed in the treatment of gynecological cancer in rats (7, 8). The BIM gene is one of the most important genes in the internal pathway or apoptosis. In addition to the known role in apoptosis induction, BIM is in the physiology of cell survival during evolution. BIM plays an essential role in the homeostasis of leukocytes and the prevention of their processes. The BIM gene is divided into three isoforms (ie, BimEL, BimL, and BimS), all of which are classified as BH3-only. The properties of pro-apoptotic-only genes, such as BIM, are connected to anti-apoptotic protein and are then allowed to form BAX pro-apoptotic and BAK to form channels on the mitochondrial membrane, which cause the release of cytochrome C and apoptosis (9).
2. Objectives
This study aimed to determine the effects of flunixin and Ginkgo biloba on the viability and apoptosis of ovarian cancer cells in the A2780s cell line.
3. Methods
3.1. Cell Culture
For this experiment, the A2780s cell line (Pasteur Institute, Iran, NCBI code: C461) was used. RPMI 1640 medium with 10% (fetal bovine serum) serum and 1% PenStrep (ie, penicillin-streptomycin antibiotic solution) were used for cell culture and then incubation. The cell density was estimated under a microscope after 3 to 4 days, and the cells were transferred to a new flask when the density reached 70 - 80%.
3.2. Drug
For this study, 100 mg/mL flunixin solution was purchased from Rooyan Darou Pharmaceutical Company, Iran. Then, 50 and 100 λ flunixin were dissolved in phosphate-buffered saline (PBS) medium for serial dilutions. Additionally, Ginkgo biloba tablets were purchased from Vitarmonil Darou Pharmaceutical Company, Iran, as 100 mg/mL. For 100, 50, and 25 λ dilutions, Ginkgo biloba was dissolved in standard saline solution.
3.3. Cell Preparation
3.3.1. IC50 Method
After 24 h of incubation under optimum conditions, for calculating the half-maximal inhibitory concentration (IC50), different doses of flunixin and Ginkgo biloba were added to each well. The well was first washed with PBS after 24 h, and then the cells were removed from the plates by trypsinizing them. Then, 50 µL of the cell solution and 50 µL of trypan blue (vital dye) were poured into the microtubule to count. Then, about 10 µL of the solution was poured on the Neobar slide.
3.3.2. Cell Culture
First, the cells were cultured in 24-well plates. Ginkgo biloba and flunixin were each added at a dose of 100 mg to target well after 24 h of cell attachment. Cells receiving only one drug, cells receiving both drugs at the same time. For the preservation of the ribonucleic acid (RNA) of the cells, cell plaques were rapidly transferred to a nitrogen tank. In the case of combination drugs, some of them were applied in a specific schedule and, after 24 h from the first drug to the second drug, regularly changed every 24 h. Moreover, the drug was added to the culture medium again.
3.4. Molecular Section
3.4.1. RNA Isolation
The isolation of RNA was performed based on the High Pure RNA Isolation Kit (Roche Life Science, Germany) guidelines. Electrophoresis was performed on an agarose gel to ensure the quality of the extracted RNA. The presence of two clear bands on the gel confirmed the health of the extracted RNA. Furthermore, optical absorption ratios of 260/280 and 260/230 nm were obtained using NanoDrop.
3.4.2. cDNA Synthesis
Complementary deoxyribonucleic acid (cDNA) synthesis was performed based on the Thermo Scientific Kit protocol. Accordingly, 1 μL RNA, 1 μL Random Hexamer, and 6 μL diethyl pyrocarbonate water were mixed, spun, and incubated in a thermal cycler for 5 min at 65°C. Then, 4 μL 5x Reaction Buffer, 2 μL deoxyribonucleotide triphosphate, and 1 μL RT enzyme were added and reached the final volume of 20 μL with deionized distilled water (DDW). The temperature-time program was performed at 25°C for 10 min, 42°C for 60 min, and 65°C for 10 min. The synthesized cDNA quality was ensured using NanoDrop.
3.4.3. Primer Design
Dedicated primers were designed by the Gene Runner Software (version 6.5.52) and blasted by NCBI (Table 1).
| Primer Name | Sequence (5’ to 3’) | Tm | Amplicon Size |
|---|---|---|---|
| GAPDH | 102 | ||
| F | TCCTCCACCTTTGACGCTG | 53.3 | |
| R | CACCACCCTGTTGCTGTAGC | 52.9 | |
| BIM | 145 | ||
| F | GAAGGCAATCACGGAGGTG | 52.7 | |
| R | AGGATCGAGACAGCAGGGAG | 53.3 |
Primer Sequence for Real-Time Polymerase Chain Reaction
3.4.4. Investigation of Gene Expression
For real-time polymerase chain reaction (PCR), SYBR Green Master Mix (Applied Biosystems, Warrington, UK) was used. Accordingly, 10 μL SYBR green, 1 μL of forward and reverse primers of each gene, and 5 μL cDNA were mixed and reached the final volume of 20 μL with DDW. The temperature-time program was performed at 95°C for 1 min, 60°C for 15 sec, and 60°C for 60 sec. The melting curve was investigated to ensure the specificity of the product. All the experiments in this study were repeated three times. Gene expression was measured by the 2-∆∆Ct method.
3.5. Statistical Analysis
After measuring the gene expression by the 2-∆∆Ct method and using Excel software (version 2.16), the data were obtained from real-time PCR, and viability assay sections were performed using SPSS software (version 25), one-way analysis of variance, Tukey’s test, and CompuSyn software (version 1.0.1). A p-value of less than 0.01 was considered the significant level.
4. Results
4.1. IC50 Method
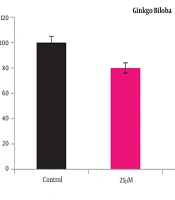
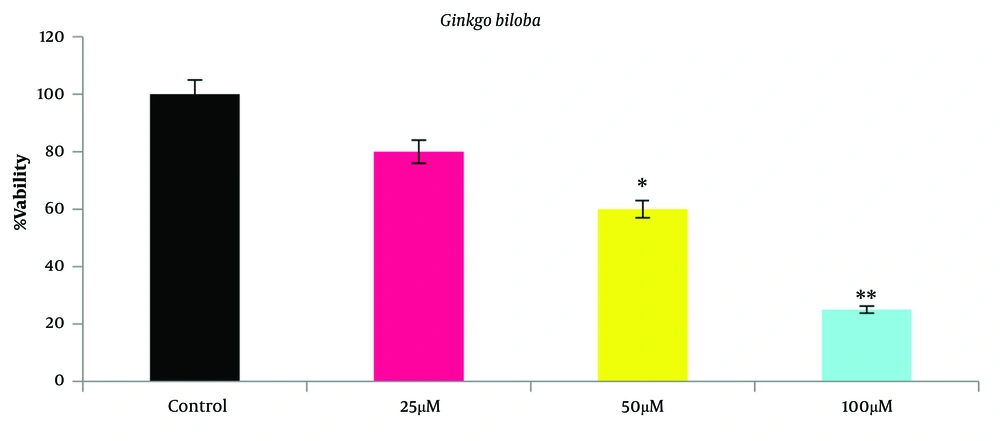
A2780 cells were cultured at 25, 50, and 100 μm Ginkgo biloba and 50 and 100 μm flunixin concentrations in 24 h. The results showed that after 24 h of incubation, the reduction of Ginkgo biloba and the survival rate of flunixin were dose-dependent (Figures 1 and 2). Accordingly, the number of living cells, compared to that of the control group (mean ± standard deviation), was significantly reduced from a concentration of 50 to 100 μm.
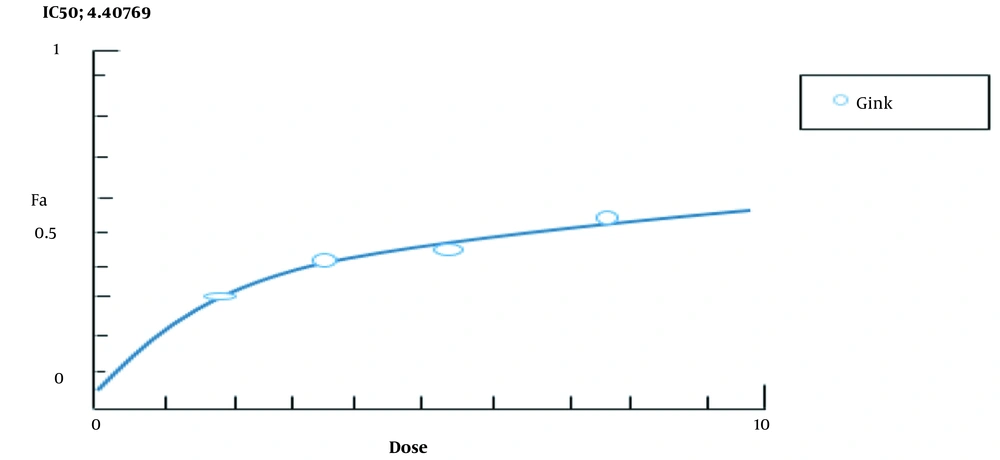
The bioavailability decreased with increasing the concentrations of Ginkgo biloba and flunixin in the A2780s cell line after 24 h of incubation. The bioavailability of both drugs was significantly reduced from 97.5% to less than 50%. According to Figure 2, IC50 for Ginkgo biloba, and flunixin was, which was followed by the experimental process using 100 μm concentration. Tukey’s test showed a significant difference between doses of 50 and 100 µm in the treatment group; however, the concentration of living cells was significantly reduced from 50 to 100 μm, compared to that reported for the control group.
4.2. Investigation of Cell Viability at Different Times
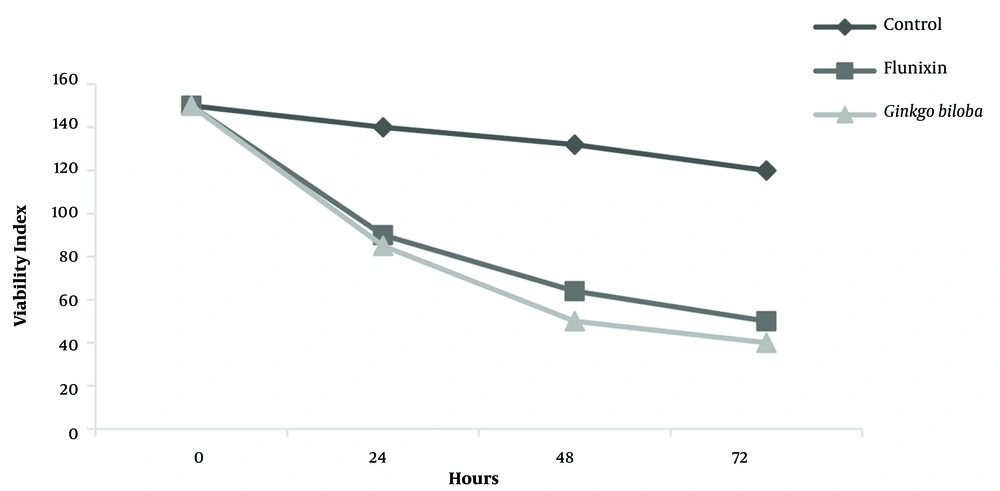
The results in Figure 3 showed that the treatment with Ginkgo biloba and flunixin affected A2780s cell viability using the best dose of the inhibitor (100 μm).
At this dose, cell viability significantly reduced in comparison to witness cells at 72 h. treatment with Ginkgo biloba. Cell viability decreased over time. Different times showed numerous differences (P < 0.01); however, the highest lethality according to Ginkgo biloba and flunixin was in 24 h. There was no significant relationship between the treatment group and Ginkgo biloba, and flunixin at 24, 48, and 72 h in Tukey’s test; nevertheless, it was significant in comparison to the control group (P < 0.01). The combination index (CI) values were determined using CompuSyn software.
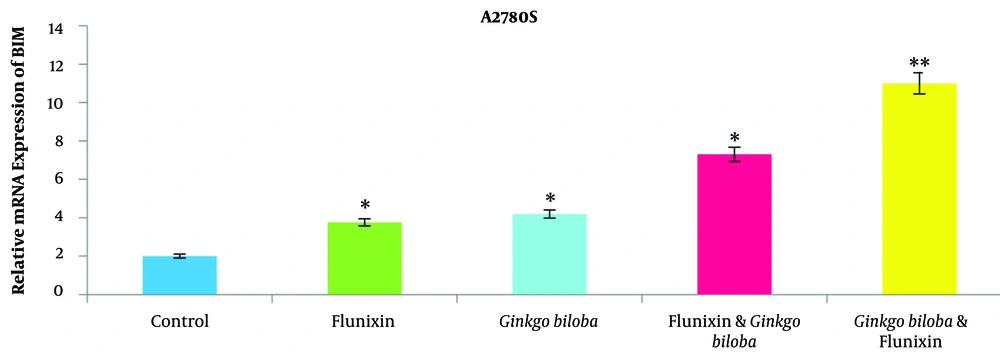
4.3. BIM Gene Expression
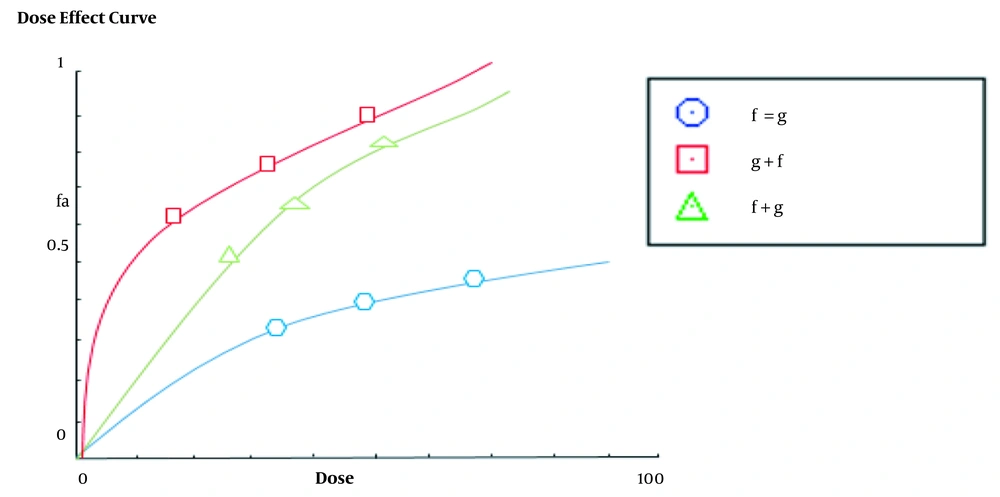
In this study, BIM gene expression was increased in the A2780s cell line treated with Ginkgo biloba and flunixin, compared to that reported for the control cells (Figure 4). The rate of expression increase in Ginkgo-biloba-treated cells for 48 h was higher than those treated with Ginkgo biloba for 24 h (Figure 4). After 24 h of treatment, Ginkgo biloba and flunixin increased BIM gene expression (Figure 4). The CI of synergistic effects between Ginkgo biloba and flunixin was analyzed using CompuSyn software. Accordingly, 100 μm flunixin had synergistic effects (CI < 7.3) with Ginkgo biloba and strong synergistic effects (CI < 11.0) with Ginkgo biloba on flunixin (Figure 5).
5. Discussion
The present study investigated the effects of flunixin and Ginkgo biloba on the ovarian cancer viability of the human A2780s cell line. In this study, flunixin and Ginkgo biloba were administered for 24, 48, and 72 h in the cell line. Cell viability decreased over time; however, most deaths occurred within 24 h (50%; Figure 3). The results showed that the use of 50 µm of flunixin and Ginkgo biloba in 24-hour cultivation could reduce the viability of these tumor cells by 25%. In addition, as the concentration increases, the lethality of the drug increases. The concentrations of 100 µm in both drugs reduced cell viability by more than 50%. The results of the present study showed the effects of flunixin and Ginkgo biloba on the A2780s cell line, which causes the expression of the BIM gene and its increase, increased over time, indicating the difference between the groups at a significant level (Figure 4).
The results showed the interaction and synergy of both drugs on the A2780s cell line, which either affects one drug and the effect of another drug after about 24 hours of effect on the cancer cell multiplied. The expression of the BIM gene in the cell lines treated with Ginkgo biloba, exposed to the drug for 48 h, significantly increased in comparison to that reported for 24 h (Figure 4 and 5). The aforementioned findings are also consistent with the results of a study performed by Jiang et al. showing that Ginkgo biloba extract could have anticoagulant and apoptotic effects on ovarian cancer cells (5). According to the aforementioned study, it can be said that Ginkgo biloba is effective as an anti-inflammatory agent and inhibitor of apoptosis and can be helpful in the treatment of ovarian cancer (9, 10). The onset of apoptosis is due to cell surface receptors, such as Fas receptors and tumor necrosis factor receptor 1 (external pathway), various genotoxic agents, metabolic toxins, or transcriptional signals (intrinsic pathway), which cause apoptosis (11).
Cytochrome C secretion and mitochondrial cleavage lead to the activation of caspase-9 and caspase-3 (12). The external pathway can bypass the mitochondrial stage, leading to cell destruction (13, 14). This protein induces apoptosis by inhibiting the anti-apoptotic members of this family and plays an essential role in tumor cell biology. Decreased BIM expression is effective in increasing cell proliferation and metastasis of cancer cells (15). Ginkgo biloba inhibits cell cycle in phase G0/G1 to phase S (14). Ginkgo biloba and its components, such as quercetin and ginkgolides, are effective in numerous cancers, such as bladder and breast cancers, by increasing antioxidant activity through various pathways (16).
Flunixin reduces anaplasia and cell proliferation by inhibiting cyclooxygenase by reducing epithelial growth factor (16, 17). On the other hand, cyclooxygenase by reducing p53 of tumor suppressor gene reduces cell arrest in the G1 stage (18). In most cancers, p53 levels in the cytoplasm increase with increasing cyclooxygenase. In addition, when inhibitors are removed from P53, it will function to kill cells (17, 18). Cyclooxygenase increases the production of an enzyme called aromatase by producing E2-type prostaglandins (18). This enzyme, known as CYP19, is one of the enzymes in cytochrome P450 and can convert endogens to estrogen. Since estrogen induces increased tumor growth (19), estrogen levels might decrease and inhibit tumor growth by the inhibition of aromatase by cyclooxygenase inhibitors. The inhibition of apoptosis by various (17, 20), such as the inactivation of tumor necrosis factor receptor (14), increased expression of anti-apoptotic protein BCL2 (20), and increased anti-apoptotic protein, prevents the mitochondrial membrane from releasing cytochrome C into the cytocell; finally, apoptosis will not occur (20, 21). In a study performed by Keramati et al., nonselective cyclooxygenase inhibitors and compounds called celecoxib, which are selective COX-2 inhibitors, were effective in the inhibition and treatment of breast cancer (10).
Since nonsteroidal anti-inflammatory drugs, such as aspirin, indomethacin, and ibuprofen, act as nonselective cyclooxygenase inhibitors and flunixin is a nonsteroidal anti-inflammatory drug, the results showed that flunixin had a positive effect on the treatment and prevention of cancer. Microscopic results obtained from drug-receiving groups showed that cancer cells are degenerating, to the point that the cells are regaining their regular order.
5.1. Conclusion
Ginkgo biloba and flunixin have an inhibitory effect on the survival of ovarian cancer cells (the A2780s cell line) in a dose-dependent and time-dependent form. Ginkgo biloba and flunixin also increase BIM gene expression in A2780s cells. The present study confirms previous studies and promises that Ginkgo biloba and flunixin have toxic effects on ovarian cancer cells, the A2780s cell line. Furthermore, Ginkgo biloba and flunixin compounds can be used to develop ovarian anti-cancer drugs.