1. Background
Mesenchymal stem cells (MSCs) and MSCs-derived insulin-producing cells (IPCs) are valuable cells for therapeutic applications. Therefore, during the past decade, researchers have tried to identify new external small molecules and regulators to improve the biological characteristics of these cells for their therapeutic applications, especially for the treatment of diabetes mellitus. Diabetes mellitus is a chronic metabolic disorder characterized by hyperglycemia and autoimmune destruction of beta-cells islets (type 1 diabetes), extensive defection and depletion of β-cells, and insulin resistance in the peripheral tissues (type 2 diabetes) (1). There are several drugs and methods to improve glycemic control and treat diabetes. In this regard, restoring pancreatic β-cells through replacement therapy by the potential therapeutic approaches, such as the induction of the β-cell regeneration or islet of Langerhans transplantation, has attracted special attention for the prevention and treatment of diabetes (1, 2).
Mesenchymal stem cells have various sources and unique characteristics, which make them one of the most important cells in regenerative medicine (3). Importantly, these cells can be converted to physiologically well functional insulin-producing β-cells (IPCs) in vitro and transplanted to diabetic patients as a source of alternative islets (4). Although the postnatal maturation of the pancreas is still unknown, Pdx1, MafA, NeuroD, preproinsulin, insulin, Glut2, and glucokinase are known as important factors for the development and function of mature β-cells (5, 6).
Nowadays, the differentiation of MSCs and formation of IPCs have been performed using treatment of the cells with specific growth factors and small molecules (7, 8). For example, Zang et al. cultured rat bone marrow-derived MSCs (rBM-MSCs) in a serum-free medium in the presence of β-mercaptoethanol, nicotinamide, and a high glucose concentration. Then, they achieved incompletely differentiated IPCs that expressed insulin (4). However, other modified protocols were used with more effective inducers, and other types of MSCs derived from different sources, which ultimately yielded favorable results (5, 9).
During the past decade, researchers have tried to identify new compounds influencing the biological characteristics of MSCs and IPCs for improving their therapeutic applications. Nowadays, medicinal herbs and their natural bioactive compounds have received significant attention in biomedical studies. Walnut (Juglans regia L.), as a well-known member of the Juglandaceae family, is one of the most broadly studied plants in both phytochemical and pharmacological aspects. Different parts of the walnut tree have been commonly used in food and dyeing industries and in medicine as a traditional remedy to treat a variety of health-related conditions. The identification of a wide range of biological constituents in the internal septum of walnut kernel and their pharmaceutical and safety properties have introduced the walnut septum as a natural herbal material with valuable properties. Phytochemical analyses have indicated that the walnut septum is a rich source of polysaccharides, phytosterols (beta-sitosterol and campesterol), and secondary metabolites like polyphenols (including gentisic acid, p-coumaric acid, ferulic acid, hyperosid, isoquercitrin, quercitrin, quercetin, epicatechin, catechin, syringic acid, gallic acid, protocatechuic acid, and vanillic acid), which are introduced to be responsible for a range of biopharmaceutical activities of the walnut septum.
Although different studies have confirmed several biological activities of the internal septum extract of walnut kernel (10-14), there is a scarcity of studies regarding its effect on the characteristics and functions of MSCs and MSCs-derived IPCs.
2. Objectives
In the present study, we investigated the effect of the aqueous decoction of the internal septum of walnut kernel (DISWK) on the rBM-MSCs cell cycle and on the expression of genes involved in insulin-producing β-cell commitment and glucose uptake in rBM-MSCs-derived IPCs.
3. Methods
3.1. Animals and Plant Materials
Four-week-old male Wistar rats were purchased from Kermanshah University of Medical Sciences (Kermanshah, Iran). Open-air sun-dried Persian walnut (Juglans regia L.) fruit was prepared from Sisakht (Kohkiluye-Boyrahmad, Iran) and authenticated by the Biology Department of Razi University (Kermanshah, Iran).
3.2. rBM-MSCs Isolation and Characterization
The Wistar rats were euthanized using chloroform asphyxiation. Then, the rat bone marrow was extracted from its femurs and tibias. To obtain rBM-MSCs, the extracted bone marrow was cultured in a Dulbecco's Modified Eagle's Medium/Low Glucose (DMEM/LG) (Gibco, UK) supplemented with 15% (v/v) fetal bovine serum (FBS) (Gibco, UK), penicillin (10 U/mL)-streptomycin (10 μg/mL) solution (Pen-Strep; Bioidea, Iran), and 2 mM L-glutamine (Sigma, Germany) at 37°C and in 5% CO2. Then, the purified rBM-MSCs at passages 3 - 5 were used to be characterized by the flow cytometry analysis of rBM-MSC-specific positive (CD44, CD90, and CD105) and negative (CD34 and CD45) markers and differentiation assays into adipocytes and osteoblasts, according to our previously established protocol for mouse MSCs (15).
3.3. Preparation of DISWK
The internal septum was separated from the walnut kernel and mechanically broken into small fragments. To prepare an aqueous decoction of the internal septum of the walnut kernel, 20 gr of small fragments of the septum were gently boiled for 30 min in 100 mL of distilled water. Then, the aqueous decoction was passed through a filter, and the filtered solution was dried in a dark and dry place at a constant temperature of 37°C. To prepare a stock solution of DISWK (5 mg/mL), 5 mg of the DISWK powder was dissolved in 1 mL of distilled deionized water and then filtered by a 0.2-μm filter under sterile conditions.
3.4. Cytotoxicity Assay
The cytotoxic activity of DISWK was evaluated by the MTT (3-(4, 5-dimethylthiazol-2-yr)-2, 5-diphenyltetrazolium bromide) (Sigma, Germany) reduction assay according to our previously established protocol (15). Briefly, the third passage of rBM-MSCs was treated with different concentrations of DISWK (0, 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, and 1 mg/mL) for 24 and 48 h. Subsequently, the MTT assay was carried out in triplicate and repeated at least four times independently to determine the cytotoxic effect of DISWK on MSCs.
3.5. Cell Cycle Analysis by PI-Staining
Cell cycle analysis was performed by the flow cytometry of the propidium iodide (PI; Sigma, Germany)-stained rBM-MSCs after treatment with DISWK. In doing so, the rBM-MSCs were treated with the 50 μg/mL concentration of DISWK for 24 and 48 h. After the incubation times, the treated cells were harvested and fixed in cold 70% ethanol. Then, the fixed cells were stained by PI (50 μg/mL) for 1.5 h at 4°C. Finally, all the samples were analyzed using FAC scan flow cytometry (Becton Dickinson, Germany).
3.6. Total Antioxidant Capacity of the DISWK-treated rBM-MSCs
To determine the effect of DISWK on the total antioxidant capacity (TAC) of rBM-MSCs, the third passage cells were cultured in a serum-free Roswell Park Memorial Institute medium (RPMI 1640) (Gibco, UK) at 37°C and in 5% CO2 for 24 h. Then, the cultured rBM-MSCs was treated with the different concentrations of DISWK (i.e., 0, 0.25, 0.5, 0.75, and 1 mg/mL) for 24 and 48 h. Next, the effect of DISWK on the rBM-MSCs was evaluated by a TAC assay kit (ZB-TAC-A48, Germany), according to the manufacturer's instructions.
3.7. Transdifferentiation of rBM-MSCs Into IPCs
The rBM-MSCs were cultured in a serum-free DMEM/high glucose (DMEM/HG) medium supplemented with nicotine amide (1 M) and β-mercaptoethanol (0.5 M) (Sigma, Germany). After 48 h, the cell culture medium was replaced with a serum-free DMEM/HG medium supplemented with nicotine amide (1 M), and then the cells were incubated for four weeks. During the transdifferentiation period, the cell culture medium was refreshed every 48 h, and the morphological changes of cells were investigated under an inverted microscope. Finally, dithizone (DTZ) staining, the microscopic observation of the islet-like cell clusters, and the expression analysis of genes involved in insulin-producing β-cell commitment were used to confirm the transdifferentiation of rBM-MSCs into IPCs.
3.8. Gene Expression Analysis
The rBM-MSCs-derived IPCs were treated with a serum-free DMEM/HG medium supplemented with 50 μg/mL of DISWK for 24 h. After the incubation time, total RNAs were extracted from treated and untreated IPCs by an animal tissue RNA isolation kit (DenaZist, Iran). Next, cDNAs were synthesized by 1 μg of the DNase I-treated total RNAs using a reverse transcription kit (Takara, Japan). Subsequently, the expression of the target genes (i.e., Pdx1, Ins1/2, Insr, Glut1, and Glut4) in DISWK-treated IPCs was evaluated by quantitative real-time PCR (qRT-PCR) using SYBR® Premix Ex Taq™ (Takara, Japan) and specific primers (Table 1). The expression of target genes was normalized to β-Actin gene as an internal control. Finally, the relative expression of the studied genes in the treated and untreated IPCs was estimated by applying the 2-ΔΔCT method (16).
| Gene & Primer | Sequence | Product Length (bp) |
|---|---|---|
| β-actin | 166 | |
| Sense | 5'-CACACCCGCCACCAGTTC-3' | |
| Antisense | 5'-GACCCATACCCACCATCACAC-3' | |
| Pdx1 | 111 | |
| Sense | 5'-GTGCCAGAGTTCAGTGCTAATCC-3' | |
| Antisense | 5'-ACTTCCCTGTTCCAGCGTTCC-3' | |
| Ins1/2 | 195 | |
| Sense | 5'-CCATCAGCAAGCAGGTCATTGTTC-3' | |
| Antisense | 5'-CGACGGGACTTGGGTGTGTAG-3' | |
| Insr | 147 | |
| Sense | 5'-GATGCCACCAATCCTTCCGTTCCC-3' | |
| Antisense | 5'-GCTGTCCTCCGCCTGCCTCTC-3' | |
| Glut1 | 190 | |
| Sense | 5'-TACTGCTCAGTGTCATCTTCATCC-3' | |
| Antisense | 5'-ATCTGCCGACCCTCTTCTTTCATC-3' | |
| Glut4 | 165 | |
| Sense | 5'-GTCTTCCTTCTATTTGCCGTCCTC-3' | |
| Antisense | 5'-TTTTGCCCCTCAGTCATTCTCATC-3' |
3.9. Statistical Analysis
All the statistical analyses were performed using SPSS version 22 and One-way analysis of variance (ANOVA) and the Tukey’s test. The data are presented as mean ± SEM. Furthermore, the fold change (FC) cut off ≥ 1.5 was considered significant for the expression of the target genes. Moreover, P-value cut-offs ≤ 0.05 and ≤ 0.01 were considered significant for the MTT and TAC assays, respectively.
4. Results
4.1. Isolation and Characterization of the rBM-MSCs
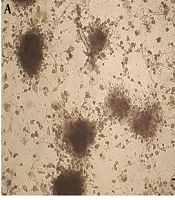
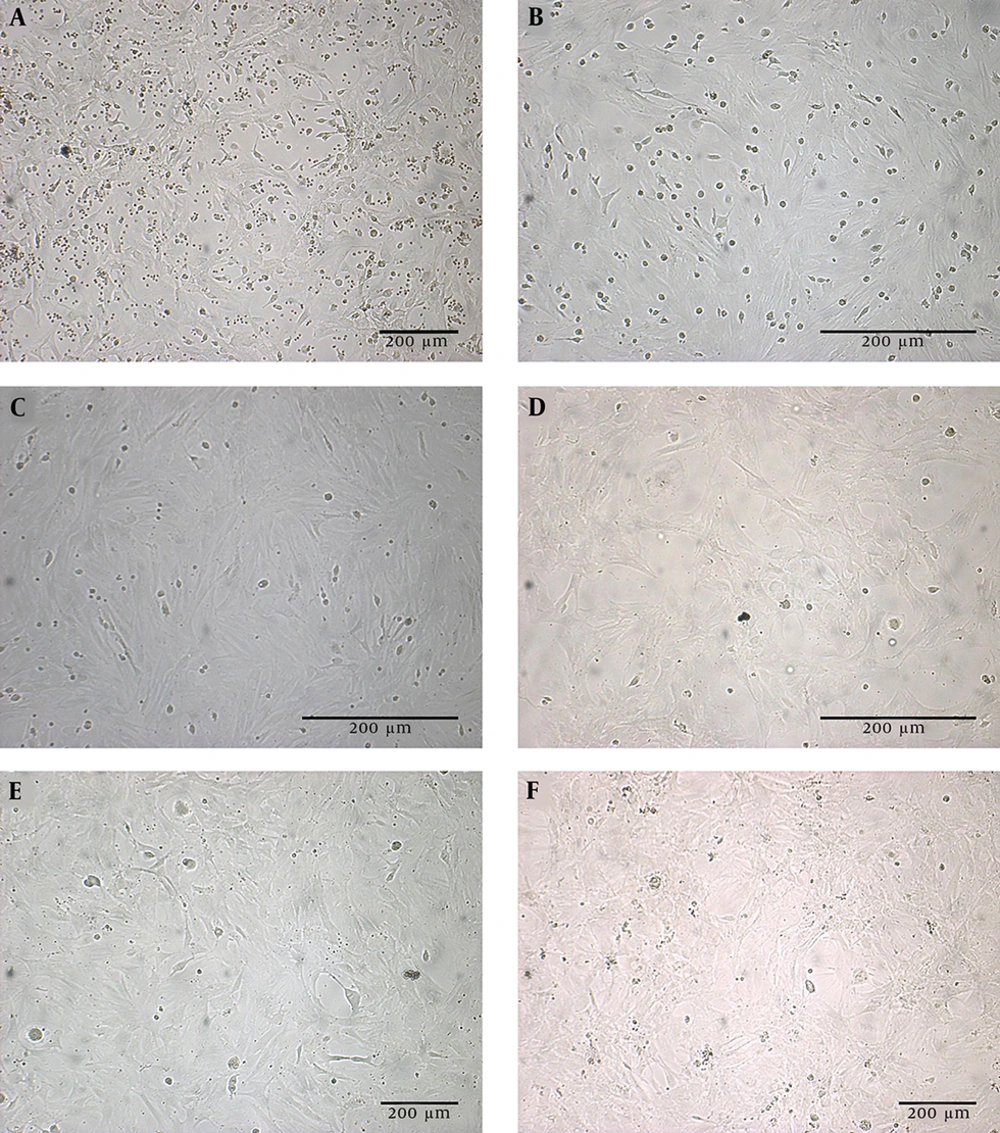
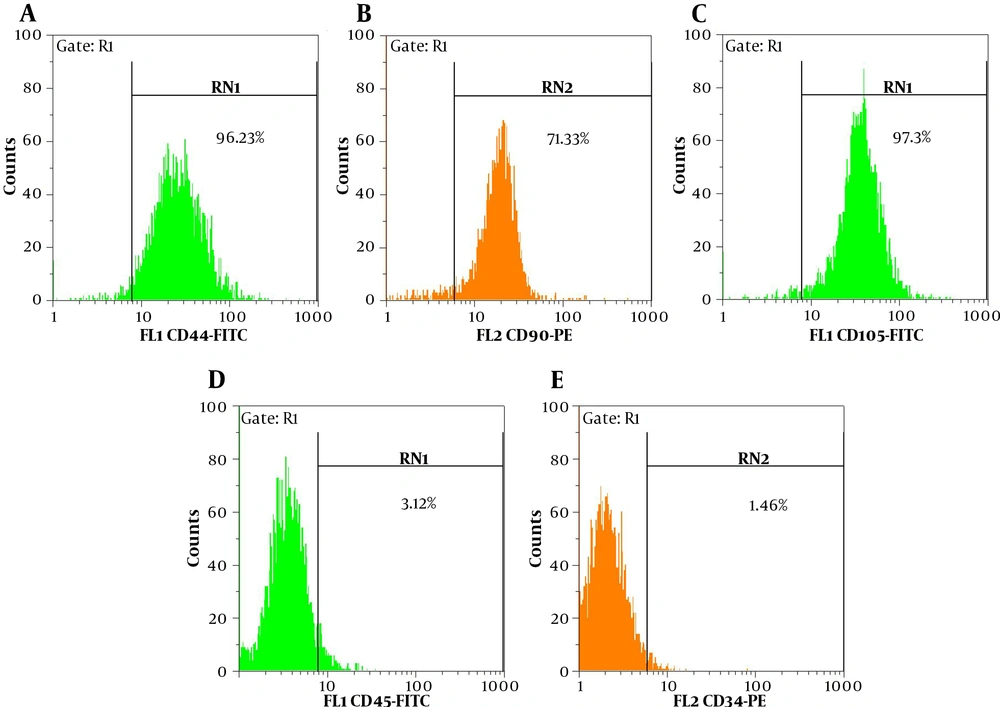
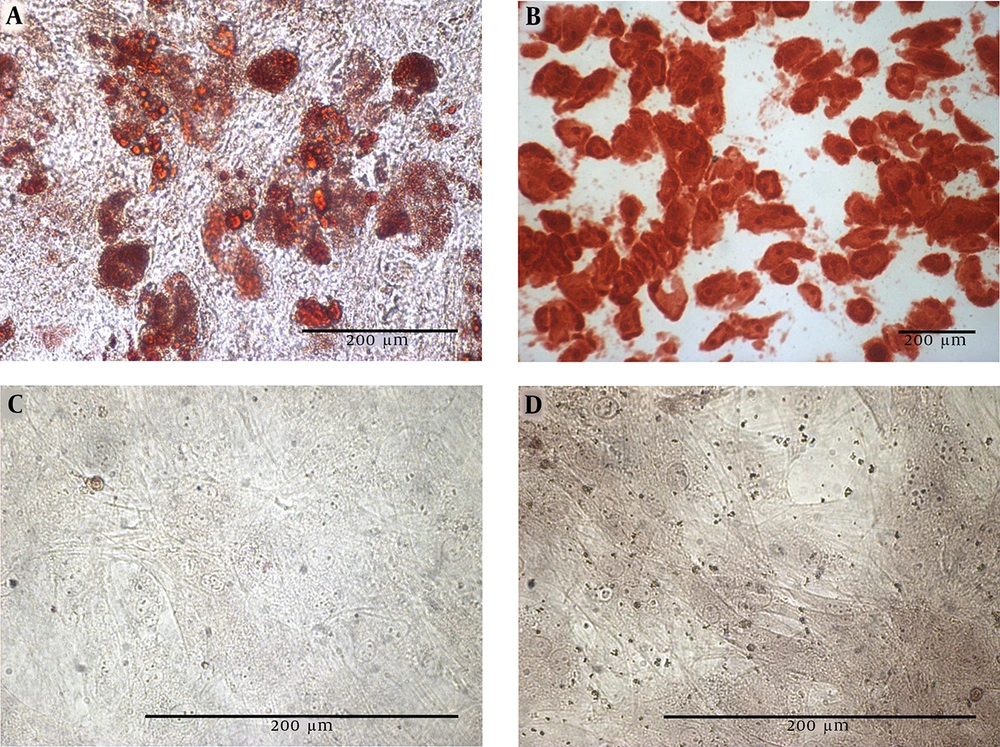
The rat BM-MSCs was isolated and expanded in vitro. Figure 1 displays a bone marrow primary culture and the subcultured rBM-MSCs at different passages. The purified rBM-MSCs were characterized for the expression of MSC-positive and -negative cell surface markers by flow cytometry. The results revealed that 96.23, 71.33, 97.3, 3.12, and 1.46% of rBM-MSCs expressed CD44, CD90, CD105, CD45, and CD34, respectively (Figures 2A-E). Moreover, the differentiated rBM-MSCs in the adipogenic- and osteogenic-inducing media demonstrated the presence of lipid vacuoles (Figure 3A) and calcium deposition (Figure 3B), which are shown in red color, in the MSCs-derived adipocytes and osteoblasts, respectively.
4.2. Cytotoxic Effect of DISWK on rBM-MSCs
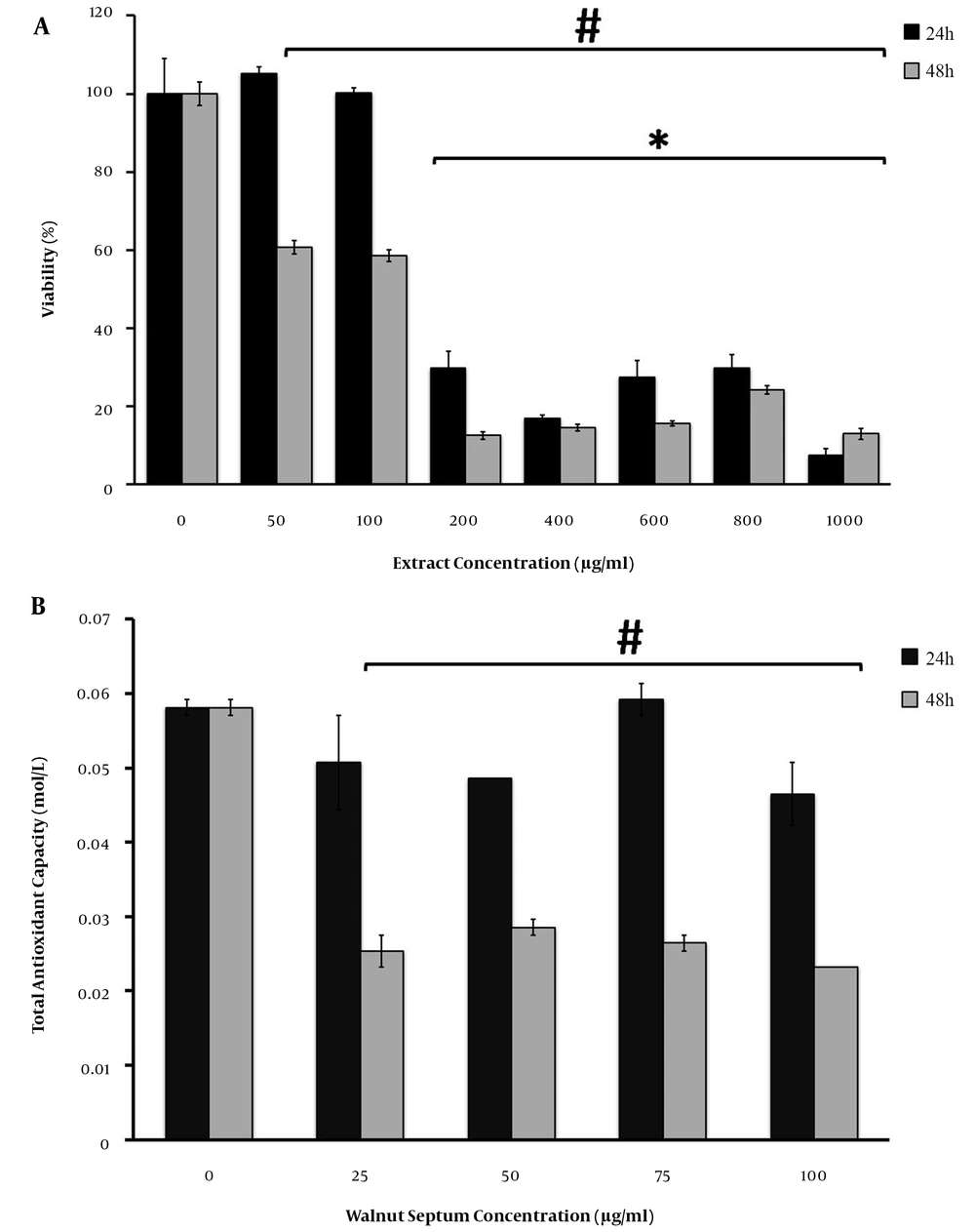
The MTT assay results indicated that DISWK induced a distinct dose- and time-dependent reduction of rBM-MSCs viability in vitro. According to the statistical analysis of the MTT assay results, the rBM-MSCs’ viability (P ≤ 0.05) reduced significantly at concentrations > 100 and ≥ 50 μg/mL of DISWK at 24 and 48 h after treatment, respectively (Figure 4A). Moreover, the half-maximal inhibitory concentration (IC50) values of the extract on MSCs were calculated < 100 μg/mL for both time points.
4.3. Cell Cycle Analysis of the DISWK-treated rBM-MSCs
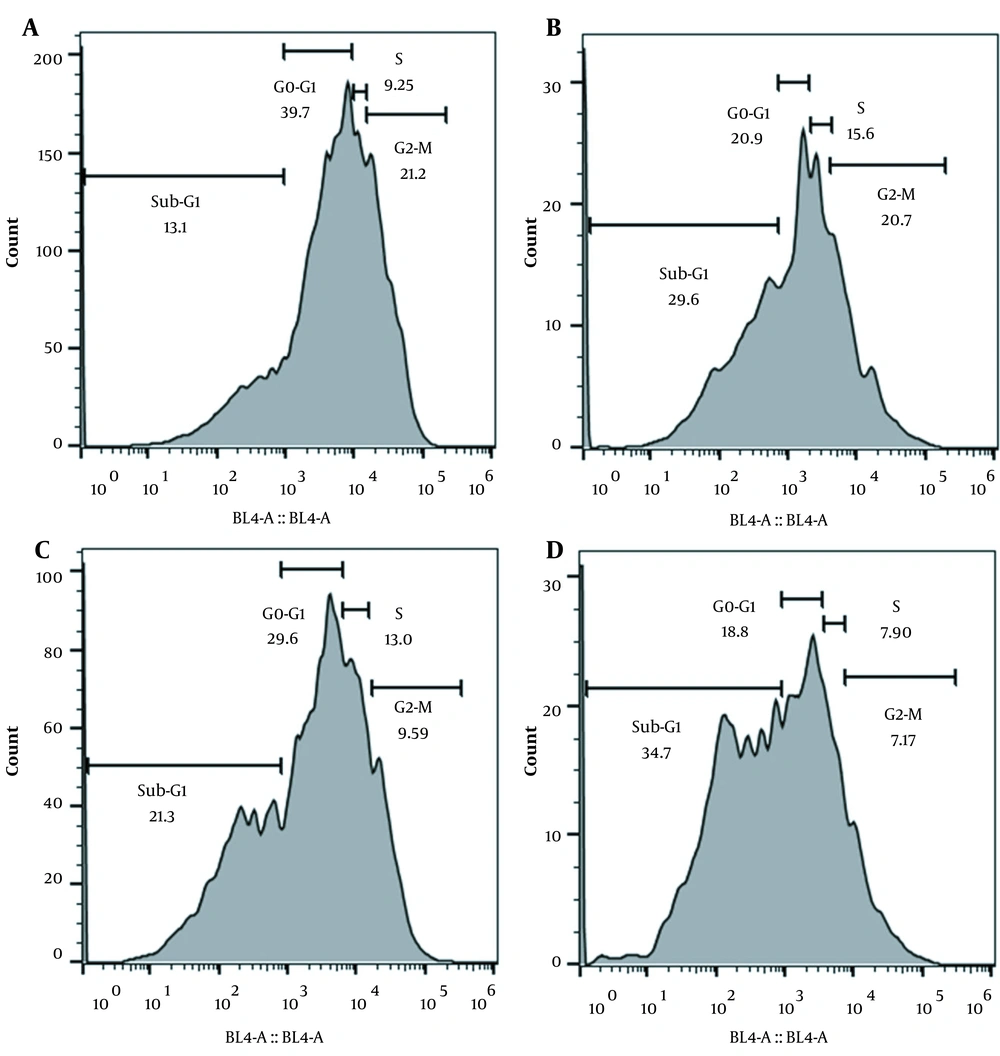
The results of cell cycle assay showed that the Sub-G1 of the cell cycle was increased by 8.2% and 5.2% in the DISWK-treated rBM-MSCs at 24 and 48 h after treatment compared with untreated control cells, respectively. Our results also indicated that although DISWK reduced the cell cycle G1-phase (10.1 %) in the rBM-MSCs at 24 h after treatment (Figure 5A), this reduction was not considerable (2.1%) at 48 h after treatment (Figure 5B), as compared with untreated controls (Figures 5C and D ). Furthermore, we found that DISWK increased the cell cycle S-phase (3.75 %) in the rBM-MSCs at 24 h after treatment. Nonetheless, the cell cycle S-phase was reduced (7.7%) in the DISWK-treated rBM-MSCs at 48 h after treatment, as compared with untreated cells. Interestingly, the results revealed that the cell cycle G2 phase was reduced by 11.68% and 13.53% in the DISWK-treated rBM-MSCs compared with controls at 24 and 48 h after treatment, respectively.
4.4. Antioxidant Effect of DISWK on rBM-MSCs
The effect of different concentrations of DISWK on the total antioxidant capacity of rBM-MSCs was investigated at 24 and 48 h after treatment. Our results indicated that DISWK did not show any antioxidant activity at 24 h after treatment (Figure 4B). However, a significant (P ≤ 0.05) TAC reduction was observed in DISWK-treated rBM-MSCs at the concentrations ≥ 25 μg/mL of DISWK 48 h after treatment, as compared with untreated control samples.
4.5. Derivation of IPCs from rBM-MSCs
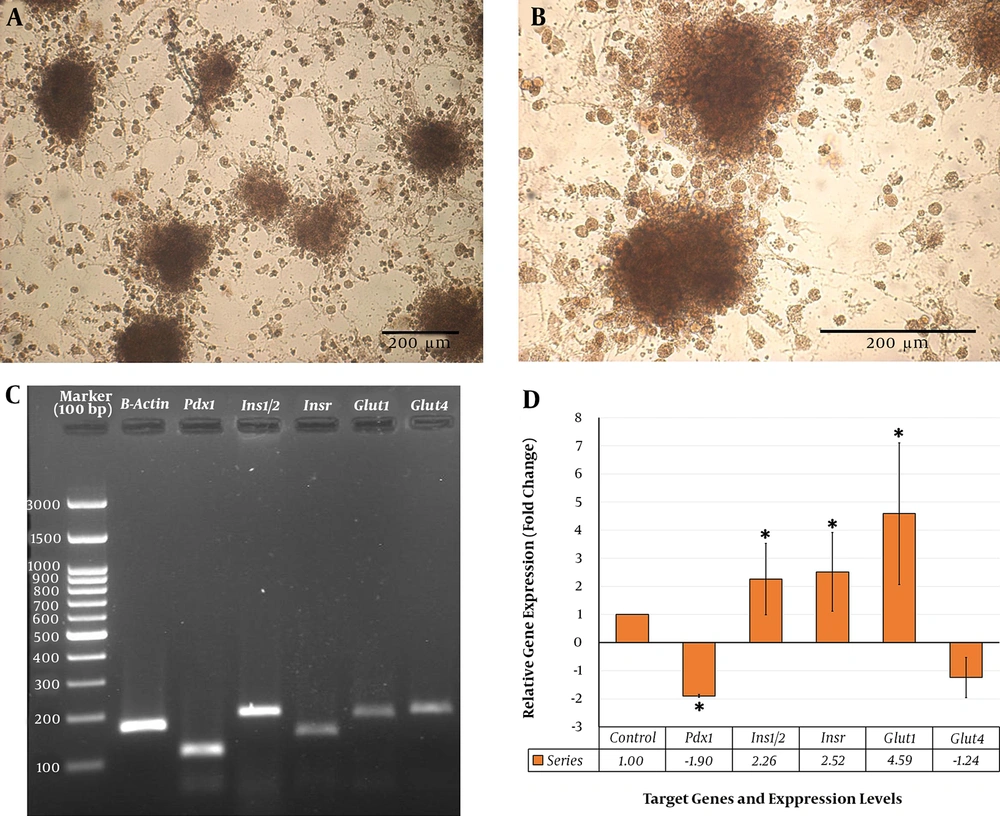
The cultured rBM-MSCs were treated with IPCs-induction media for 28 days. The morphological changes in rBM-MSCs and the production of islet-like clusters were started after the treatment of cells with the IPCs-induction media (Figure 6A). At the end of the transdifferentiation period, DTZ staining, which selectively stains pancreatic β-cells, followed by microscopic observations demonstrated crimson red color in islet-like cell clusters (Figure 6B). Furthermore, conventional RT-PCR demonstrated the expression of the genes involved in insulin-producing β-cell commitment and glucose uptake, including Pdx1, Ins1/2, Insr, Glut1, and Glut4, in the cultures containing the islet-like cell clusters (Figure 6C).
4.6. Gene Expression Changes in the DISWK-treated IPCs
The results of our qRT-PCR showed that DISWK significantly increased Ins1/2 (2.26 fold), Insr (2.52 fold), and Glut1 (4.59 fold) genes expression in the rBM-MSCs-derived IPCs compared with untreated cells. However, it significantly reduced the expression of Pdx1 gene (1.9 fold) in IPCs (Figure 6D). Furthermore, our results indicated that DISWK slightly, and not significantly, decreased Glut4 gene expression (1.24 fold) in the IPCs.
5. Discussion
This is the first attempt to investigate the impact of DISWK on the survival, cell cycle, and total antioxidant capacity of rBM-MSCs. The results indicated that DISWK significantly reduced the rBM-MSCs viability in a dose- and time-dependent manner. In accordance with our results, Rusu et al. demonstrated that exposure of human lung adenocarcinoma A549 and human breast cancer T47D-KBluc cells to walnut septum extract (WSE) resulted in a dose-dependent drop in their cellular viability. However, it did not influence human breast cancer MCF-7 and normal human gingival fibroblasts (HGF) cells (17). Moreover, they showed the antioxidant effect of WSE on all four cell types in a D-galactose-induced ageing model and naturally aged rats (17, 18).
In this regard, several phytochemical studies also demonstrated that the walnut septum is a rich source of polyphenols, including ellagitannins, catechin, epicatechin, quercetin, and gallic acid, which are introduced to be responsible for its high antioxidant property (14, 17) Despite these findings, our results indicated that DISWK reduced the TAC of rBM-MSCs 48 h after treatment. Therefore, we suggest that contrasting results may be related to the type of sample, the extraction method, the thermal inactivation of DISWK polyphenols, or the presence of antioxidant inhibitory compounds in the extract. However, further phytochemical and functional analyses are required to confirm this conclusion.
Our study also elucidated that DISWK suppressed the rBM-MSCs cell cycle, especially at S and G2 phases. In this regard, several studies also reported that WSE suppresses the proliferation of cancerous cells (17, 19, 20). Functionally, the walnut septum phenolic compounds are introduced to be responsible for its antiproliferative effects (17, 21). Therefore, we propose that DISWK may have sparingly water-soluble polyphenols (e.g., epicatechin, catechin, quercetin, syringic acid, gallic acid, protocatechuic acid, and vanillic acid) and heat-resistant bioactive constituents, which inhibit the rBM-MSCs cell cycle at the S and G2 phases. According to the previous studies and ours, we declare that although the walnut septum can be used as an antiproliferative agent against diverse cancerous cells, it also suppresses the cell cycle of normal MSCs. Therefore, we conclude that further functional investigations are warranted to find the exact molecular effects of the walnut septum and its constituents on the characteristics of cancerous and normal cells.
In the present study, the effect of DISWK was also evaluated on the expression of genes involved in pancreatic β-cell commitment and glucose uptake in IPCs. Growing evidence suggests that WSE can reduce blood glucose level (10-12). Although Javidanpour et al. indicated that the leaf and fruit peel of walnut increased β-cell number in the pancreas of streptozotocin-induced diabetic rats (11), Dehghani et al. reported that WSE did not have any effects on the pancreatic structure (22). In this regard, our study also exhibited significant overexpression of Ins1/2 and Insr genes in the DISWK-treated IPCs. Moreover, we evaluated the expression of Glut1 and Glut4 genes in the DISWK-treated IPCs. GLUT1 is a uniporter that uptakes glucose in GLUT2-deficient insulin-releasing β-cells (23) and plays a role in the glucose-dependent insulin secretion by β-cells (24). It cooperatively acts with other isoforms, especially GLUT4, in insulin-sensitive tissues, including fat and muscle (25, 26). Moreover, two enhancer elements have been identified in the mouse Glut1 gene responsive to insulin, growth factor, and oncogenes (26).
In the present study, although DISWK had no significant effect on the expression of Glut4 gene in the DISWK-treated IPCs, it overexpressed Glut1 in the cells. Therefore, we conclude that DISWK may induce glucose uptake through the induction of Ins1/2, Insr, and Glut1 genes expression in IPCs. In addition, according to the previous studies and ours, we suggest that DISWK may promote Glut1 expression through stimulating the expression of Ins1/2 gene in IPCs; however, further functional studies are needed to confirm it.
In this study, Pdx1 gene expression was also investigated in the DISWK-treated IPCs. PDX1 is a transcription factor required for pancreatic β-cells development and functionality (27). Our results showed that DISWK significantly reduced Pdx1 gene expression in IPCs. Therefore, we conclude that although DISWK may induce glucose uptake through the upregulation of Ins1/2, Insr, and Glut1 genes in IPCs, it may influence the insulin-producing β-cells characteristics by inhibiting the expression of the genes involved in pancreatic development, such as Pdx1. Considering the prominence of these effects, further studies should be conducted to investigate these priority areas more in-depth, especially at protein level for Ins1/2, Insr, and Glut1 genes.
Finally, we also suggest that polysaccharides, phytosterols, secondary metabolites like sparingly water-soluble polyphenols (i.e., phenolic acids, flavonoids, and condensed tannins), and heat-resistant constituents may be key compounds responsible for the molecular effects of DISWK on the MSCs and IPCs in this study. However, according to the previous studies and ours, it is worth noting that the type of sample and extraction method may influence the WSE constituents’ profile and effects on cells. Therefore, further phytochemical and functional analyses are desirable to find the potential effective compounds of DISWK and their possible molecular effects for improving the therapeutic applications of MSCs and IPCs in medicine.
5.1. Conclusions
In sum, our results indicated that DISWK significantly reduces the viability and TAC of rBM-MSCs and suppresses their cell cycle. Moreover, DISWK may help in glucose uptake by IPCs through the activation of Ins1/2, Insr, and Glut1 genes expression in these cells. However, it may influence pancreatic β-cells commitment and functionality via inhibiting Pdx1 gene expression. Altogether, the results of this study provide a good perspective for further investigations to evaluate the impact of walnut septum on MSCs and MSC-derived IPCs and to determine the potential effective compounds of DISWK and their possible molecular effects for improving their therapeutic applications in medicine and the treatment of human diseases, such as diabetes mellitus.