1. Background
Currently, the coronavirus disease 2019 (COVID-19) infection is threatening human health worldwide, causing acute damages in the vital organ systems (lungs, kidneys, and heart) (1). Coronavirus is divided into 46 different species and has been detected in animals and humans (2). After December 2019, COVID-19 infection increased in the world and caused concerns about the probable effect of COVID-19 on reproductive organs and fertility of males. It was reported that over 25 viruses could enter human semen and negatively affect spermatozoa or male fertility (3), such as herpes simplex virus (HSV) and human immunodeficiency viruses (HIV) (4). It is critical to know whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) could have the same effect on males; and this was not answered in a preliminary investigation (5). COVID-19 might induce male infertility by direct viral replication and viral dissemination-induced cytopathic effects in the testis and indirect immunopathology-caused male fertility harm (6). Findings from some studies show that the coronavirus can directly affect testicular tissue and some sperm parameters by altering the expression pattern of the angiotensin-converting enzyme 2 (ACE2) gene (7, 8). In fact, seminiferous duct cells, spermatogonia, Leydig cells, and Sertoli cells being four testis-specific cell types, express this enzyme (8). In a study by Nikolayeva et al., it was found that the ACE enzyme is located in sperm, mostly in the post-acrosomal region, neck, and middle of normal sperm (7). ACE expressed in sperm binds glycosyl phosphatidyl inositol (GPI) proteins to the zona pellucida region, which ultimately leads to the fertilization process (9). Furthermore, previous studies (10, 11) have demonstrated that ACE activity is associated with human male fertility. In addition, there is also the hypothesis that binding SARS-CoV-2 to the ACE2 receptor increases ACE2 activity in sperm cells and the side effects in fertilization owing to abnormal functions of sperm cells (8, 12, 13). Various studies have shown that the virus and its infections reduce the rate of sex hormones (14, 15). Because testosterone is one of the most important hormones in male reproduction, the reduction of its level will have adverse effects on male fertility and germ cell survival (16). Thus, changes in reproductive hormone levels caused by COVID-19 infection can reduce male fertility (15).
2. Objectives
This study examined the impact of COVID-19 on sperm function, reproductive hormone, and total antioxidant capacity (TAC), which is closely involved in this process.
3. Methods
3.1. Patient Selection
In this case-control study, a total of 100 males referred to the Infertility Treatment Center of Qom, Iran, were enrolled from May 20 to September 24, 2020. All the 60 eligible COVID-19 patients were willing to participate in the study. All cases gave a semen sample after completing written informed consent. Subjects having a positive nasopharyngeal swab test for COVID-19 (ESwab collection kit; Copan) and positive Immunoglobulin (Ig) M and IgG antibodies were considered positive for COVID-19 (17). The control group (n = 40) included healthy participants without reported andrologic pathology. The age of subjects in the control group was similar to that of the COVID-19 group (mean age: 20 - 45 years). The Ethical Review Committee of Qom University approved all the study protocols (IR.Qom.REC.1399.030). The eligibility criteria of enrolling male inpatients for COVID-19 and control groups were: (1) age over 18 years; (2) having offspring through natural pregnancy or without offspring due to specific problem of one’s wife; (3) lack of receiving infertility treatment such as drugs or assisted reproductive techniques; (4) lack of history of diseases affecting spermatogenesis (obesity, diabetes, cryptorchidism, varicocele, testicular torsion, mumps, genital tract infection, exposure to environmental chemicals, etc.).
3.2. Assessment of COVID-19 RNA
The native and processed sperm sample was centrifuged for 1 min at 3.500 rpm. RNA was extracted from 200 mL supernate by the EZ1 Virus Mini Kit v2 (Qiagen) according to the manufacturer’s instructions. Then, 60 mL was eluted from the 200 mL starting material, and 5 mL of the eluate was examined by reverse-transcription polymerase chain reaction (RT-PCR) using the TaqMan technique. A 113-base-pair amplicon in the E-gene of COVID-19 was amplified and detected, as previously described with minor modifications (17). An ABI 7500 FAST sequence detector system (PE Applied Biosystems) was used to perform RT-PCR. Thermal cycling was performed at 55°C for 10 min for reverse transcription, followed by 95°C for 3 min and then 40 cycles of 95°C for 15 s, 58°C for 30 s. The LightMix Modular SARS, Wuhan CoV E-gene (Cat. no. 53-0776- 96), and the LightMix Modular EAV RNA Extraction Control were utilized. In addition, the AgPath-ID One-Step RT-PCR Kit (Applied Biosystems cat. no. 4387391; DNA-standard plasmid pEX-A128-nCoV2019-E-gene) was used.
3.3. Assessment of COVID-19 Antibody
In this study, an ELISA-based antibody method was used to measure IgG (GLKT1032 Novel Coronavirus COVID-19 IgG ELISA Kit, Germany) and IgM (GLKT1033 Novel Coronavirus COVID-19 IgM ELISA Kit, Germany) levels in accordance with manufacturer’s instructions. Hence, 50 µL serum was assessed by the fully automatic EuroImmun Analyzer I-2 P (EuroImmun Medizinische Labordiagnostika, Germany).
3.4. Assessment of Sperm Quality
A semen sample of each participant was obtained by means of masturbation and ejaculated directly into non-cytotoxic sterile containers. Freshly gathered semen was processed within 1 hour of ejaculation to analyze sperm parameters in accordance with the 2010 World Health Organization (WHO) semen analysis criteria (18). Eosin-nigrosine staining was used to analyze the viability of sperm (eosin and nigrosine solutions 1 and 10%, both from Merck, Germany). In this staining, the dead sperm changed to red, and the living sperm remained colorless (19).
3.5. Assessment of DNA Fragmentation
Sperm Chromatin Dispersion (SCD) was used to assess DNA fragmentation (20). DNA fragmentation was examined based on the presence of an aura around the nucleus and its size. Then, 200 sperms were examined to evaluate the amount of DNA fragmentation.
3.6. Assessment of Reproductive Hormone and Total Antioxidant Capacity
The levels of follicle-stimulating hormone (FSH; mIU/mL, Cat.N.DE1288), luteinizing hormone (LH; mIU/mL, Cat.N.DE1289), and total testosterone (TT; ng/mL, Cat.N.DE1559) were calculated by ELISA method and according to the manufacturer's instruction immunoassay (Demeditec Diagnostics GmbH, Germany). The level of TAC in seminal plasma was evaluated according to the instructions of the manufacturer’s kit (CAT No. ZX‐44109‐192, Zell Bio GmbH, Germany).
3.7. Statistical Analysis
The data were analyzed and expressed as mean values ± standard deviation. SPSS software version 21 (SPSS, Inc., USA) was utilized to proceed data. Groups were compared by unpaired sample t-test and Mann-Whitney test for parametric and nonparametric data, respectively. Values were statistically significant when P-value was < 0.05.
4. Results
Subjects suffering from COVID-19 infection had a statistically significant impairment of sperm quality (sperm concentration, sperm motility, sperm viability, and normal sperm morphology) compared to those recovered in the control group. COVID-19 positive cases had significantly lower antioxidant capacity as well as significantly higher sperm DNA fragmentation than subjects in the control group (P < 0.05) (Table 1 and Figure 1).
| Parameters | COVID-19 Positive Individuals | Control Individuals | P-Value |
|---|---|---|---|
| Age (y) | 38.2 ± 9.9 | 36.4 ± 13 | 0.661 |
| BMI (kg/m2) | 24.6 ± 2.9 | 23.6 ± 2.6 | 0.521 |
| Volume (mL) | 2.8 ± 0.9 | 3.1 ± 1.0 | 0.502 |
| Sperm concentration (106/mL) | 70.9 ± 63.9 | 105.9 ± 41.1 | 0.001 |
| Total motility (%) | 48.1 ± 28.0 | 73.1 ± 27.7 | 0.001 |
| Normal morphology (%) | 7.33 ± 1.88 | 12.1 ± 2.5 | 0.001 |
| DNA fragmentation (%) | 16.45 ± 2.55 | 11.22 ± 3.22 | 0.01 |
| Viability (%) | 61.52 ± 8.34 | 87.33 ± 9.77 | 0.001 |
| TAC (IU/mL) | 2.55 ± 1.33 | 4.56 ± 2.01 | 0.001 |
a Values are expressed as mean ± SD unless otherwise indicated.
b Statistical analysis according to Mann-Whitney U test for nonparametric distribution (P < 0.05).
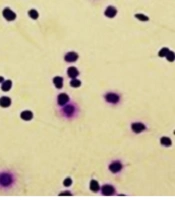
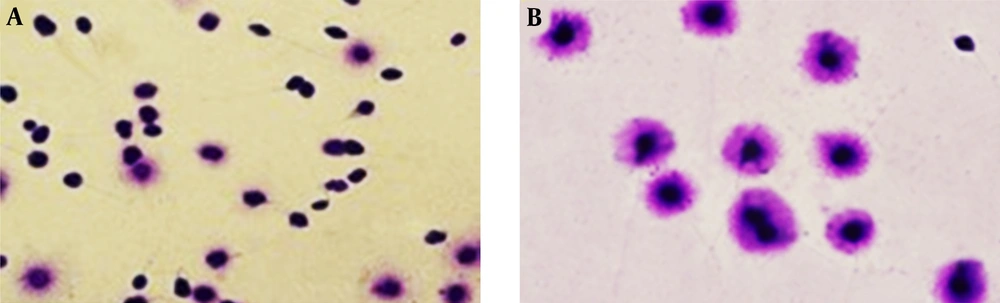
View of DNA fragmentation based on halo formation in different groups (magnification × 100). A significantly higher sperm DNA fragmentation in COVID-19 positive, A, fertile males group; B, compared to the subjects in the control group. Sperm nucleus with large halo (without DNA fragmentation), sperm nucleus with small halo (with DNA fragmentation), and sperm nucleus without halo (with DNA with severe damage).
Serum LH and testosterone were increased in COVID-19 patients in comparison to the control group (P = 0.001). There was no difference in serum FSH levels of the two groups (P = 0.34). Testosterone hormone level (P = 0.001) and the ratios of T/LH (P = 0.001) and FSH/LH (P = 0.001) were significantly lower in the COVID-19 group (Table 2).
Abbreviations: FSH, follicle stimulating hormone; LH, luteinizing hormone.
a Values are expressed as mean ± SD unless otherwise indicated.
b Statistical analysis according to unpaired sample t-test and Mann-Whitney test (P < 0.05).
c Significant difference.
Serum COVID-19 viral load had no significant correlation with semen volume (r = 0.029, P = 0.8). Significantly negative correlations were observed between serum COVID-19 viral load and sperm concentration (r = -0.431, P = 0.001), sperm motility (r = -0.603, P = 0.001), sperm viability (r = -0.750, P = 0.001), normal sperm morphology (r = -0.500, P = 0.001), and seminal TAC (r = -0.355, P = 0.005). In addition, significantly positive correlations existed between serum COVID-19 viral load and sperm DNA fragmentation index (r = 0.778, P = 0.001) (Table 3).
Abbreviations: TAC, total antioxidant capacity; DFI, DNA fragmentation index.
a Significant difference.
5. Discussion
The COVID-19 pandemic may pose a risk of infertility in males due to SARS-COV-2 (21). The higher prevalence and mortality rates from this disease in males might be due to hormonal factors, genetics, and high-risk health-related behaviors (2, 22). This infection directly or indirectly affects the male reproductive system, and it has a negative effect on male reproductive health and causes spermatogenic defects (15). The direct impact of the COVID-19 virus on the male reproductive system is through binding of this virus to ACE2, as a receptor to enter human cells (23). It has been reported that ACE2 is clearly present in the prostate, testes, epididymis, and semen of humans. This is due to the development of the testicles with age puberty, germ cell maturation, epididymal regulation, and electrolyte balance; finally, it is related to sperm capacity (24). A study found that ACE2, angiotensin 1 - 7, and MAS receptors are expressed in Leydig and Sertoli cells, indicating their possible role in steroidogenesis and spermatogenesis (25).
The presence of different viruses in the male reproductive system increases the risk of sexually transmitted infections (STIs) (26). It can also affect male fertility by being infected locally or through spermatogonia stem cells (27). Although it is not clear how much these viruses are present in semen (28), it is extremely important to assess the presence of COVID-19 RNA in semen samples because even a minor risk is not reasonable in treating healthy couples for infertility reasons (29, 30). In this study, COVID-19 RNA did not exist in the collected semen samples. Various factors cause the virus to die in the semen. Any change in the blood‐testis barrier triggers the response of inflammatory mediators and stops the immune system (31). Despite reports of transmission of COVID-19 virus through semen samples, there is no evidence of virus infection through these samples (32).
We investigated the adverse effect of COVID-19 infection on sperm parameters. The adverse impact on sperm is indicated via reduced concentrations and sperm viability, alterations in morphology, diminished motility, enhanced DNA fragmentation and significantly decreased TAC in those with good reproductive function and concomitant COVID-19 infection compared to non-infected cases. The main mechanisms of COVID-19 virus affecting sperm function and parameters can be summarized as follows: the COVID-19 virus uses the ACE2 receptor to enter human cells, which is initiated by the intramembrane serine protease TMPRSS2. Both ACE2 and TMPRSS2 receptors are expressed in the testes (33). Binding of COVID-19 virus to ACE2 can lead to an overgrowth of angiotensin II, which leads to a strong inflammatory response with dysfunction of Leydig and Sertoli cells, resulting in decreased sperm viability and function (34). Because angiotensin II is known to stimulate sperm, excess levels of this enzyme, followed by excessive ACE2, can cause inflammation and affect sperm function, especially when the inflammation in the testicles is not localized (34).
On the other hand, TMPRSS2 can break down ACE2 into amino acids 697 to 716 and facilitate the entry of the COVID-19 virus. The ACE2 gap is expected to reduce sperm viability and function following exposure to the virus, leading to loss of fertility (35). Our results are similar to some recent reports describing viral infections negatively affect semen parameters, including the semen volume, number of spermatozoa, and sperm total motility. One prominent example of the adverse effects of this virus includes disarrayed spermatogenesis (27). One study examined the effect of SARSCoV-2 viral infection on semen analysis (15). The study showed that sperm quality (sperm count and motility) was significantly lower in patients with moderate viral infection.
In another study, the hormone profile of COVID-19 patients was compared to that of healthy males with normal fertility. While there was no statistical change in serum FSH levels of the COVID-19 group, serum LH level was significantly enhanced, and testosterone (T) and testosterone/LH and FSH/LH ratios were significantly reduced.
Androgens have very complicated relation with COVID-19. Many studies show a strong impact of testosterone on the risk of COVID-19 infection (36). Gonadotropin-releasing hormone-releasing neurons secrete GnRH from the hypothalamus, which releases FSH and LH-stimulating hormone from the pituitary gland. Low levels of GnRH reduce FSH and LH, resulting in dysfunction of Sertoli and Leydig cells (37). A study showed that in COVID-19 patients, serum LH levels were significantly higher, but testosterone/LH and FSH levels decreased, suggesting hypogonadism (38). Overall, testosterone/LH ratio decreases in patients with COVID-19, indicating potential subclinical damage to male gonadal function. In addition, activation of the hypothalamic-pituitary-gonadal axis and subsequent changes in hormone concentrations have an essential role in decreasing sperm quality (39).
Despite the nature of the relation between testosterone and COVID-19 disease, an andrological examination, evaluation of sperm parameters, and hormonal evaluation are necessary during the diagnosis for several months. However, COVID-19 could have a pathophysiological effect on the testes and significantly reduce the ratio of testosterone to LH by active infection of COVID-19, indicating a significant effect on the response of Leydig cells to LH stimulation (40).
5.1. Conclusion
COVID-19 can negatively affect spermatogenesis and male fertility. Even in subjects under treatment, it is impossible that the virus exists in semen, but the rise in body temperature associated with the disease can be impaired spermatogenesis. Thus, as a preventive measure, clinical evaluation and hormonal and semen parameters in individuals suffering from this viral disease are recommended during infection, especially in severe cases. Unfortunately, initial studies have limitations such as small sample size, testing methods, and disease progression.