1.Background
Gastric cancer is one the highly prevalent cancers in the world, with a high rate of mortality (1). This cancer was observed in developing countries, especially in East Asia, mostly. The tumor was originated from the mucus-producing cells in stomach. Since the early stages of the disease are asymptomatic, the diagnosis is usually made at the advanced stage of the tumor. This limits the success and efficacy of surgery or chemotherapy (2, 3). In recent decades, molecular targeted therapy for cancer has drawn attention to enhance selective anticancer therapy. Molecular targeted inhibitors efficaciously mark specific overexpressed molecules in pathways related to tumorigenesis (4). Long non-coding RNAs (lncRNAs) are lengthy transcripts and do not code functional proteins. LncRNA genes are transcribed in a variety of human tissues and have been connected with vital biological pathways such as cell growth and death (5). It has been well established that dysregulated expression of lncRNAs may be connected with human diseases (6-8). These lengthy and non-coding transcripts drive tumorigenesis as well (5, 6).
HOX antisense intergenic RNA (HOTAIR) is one of the well-cited lncRNAs whose expression has been changed in some of the human cancers like breast, liver, ovarian, and pancreas (9). Previously published studies reported that HOTAIR is highly upregulated in gastric cancer and promotes metastasis. The HOTAIR expression promotes epithelial-mesenchymal transition (EMT), and its inhibition can reverse the EMT signaling (10). In gastric cancer cells, HOTAIR silencing negatively affects cell proliferation and invasion in vitro and in vivo (11). Although the essence and relevance of HOTAIR with cancers have been discovered in many studies, the underlying molecular mechanisms are mostly unidentified. Accordingly, the present study evaluated the consequence of HOTAIR silencing on the expression of fibronectin 1 (FN1) and claudin-4 (CLDN4) in AGS cellular model of gastric cancer. Both proteins mediate cells to the extracellular matrix adhesion and cell migration, growth and differentiation as well. Increased expression levels of FN1 and CLND4 are linked with poor prognosis in gastric cancer. It is necessary to mention that this is the first time that the effect of HOTAIR silencing was studied on expression levels of two critical factors of EMT.
2. Methods
The current study was part of an M.Sc. thesis written by P.B., which was approved by Ethical Committee of Islamic Azad University Tehran Medical Sciences, Research Affairs, Tehran, Iran University (Codding number: 13621930802269 and code of ethics: IR.IAU.PS .REC.1396.72).
2.1. Cell Lines and Culture Condition
An AGS cell line was considered a cellular model of gastric cancer purchasing from the National Cell Bank of the Pasteur Institute, Tehran, Iran. The AGS cells were fed with RPMI-1640 medium (Life Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin (Life Technologies). All cells were incubated at 37°C and 5% CO2. The AGS cells were sub-cultured every 2 - 3 days.
2.2. HOTAIR siRNA Transfection
Human HOTAIR (ID, 100,124,700) was silenced by means of specific siRNAs (GenePharma, China) as follows: HOTAIR, 5′-UAACAAGACCAGAGAGCUGUU-3′; negative control (NC), 5′-TTCTCCGAACGTGTCACGT-3′. The AGS cells were seeded in 12-well plates (12 × 104 cell/well) and transfected with 50 ng siRNA duplexes using Lipofectamine 2,000 (Invitrogen, USA) based on user guide. Cells were transfected with Lipofectamine without siRNA were considered mock. Cells were harvested for RNA extraction following 48 h of transfection.
2.3. RNA Extraction and Real-time Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted using RNX-Plus solution (SinaClon, Iran) followed by DNase I digestion (Thermo Fisher Scientific, USA). The pull-out RNAs were reverse transcribed into the complementary DNA (cDNA) through Prime ScriptTM RT reagent kit (Takara, Japan). The information about primers for specific genes and internal control gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is summarized in Table 1. Real-time PCR was done using the SYBR green Premix Ex TaqTM II (Takara, Japan). The relative gene expression was calculated with 2−ΔΔCt method.
| Primer | Forward Sequence 5'–3' | Reverse Sequence 5'–3' | Amplicon Size (bp) |
|---|---|---|---|
| FN1 | TCAACTCACAGCTTCTCCAA | CACGACCATTCCCAACACAC | 153 |
| CLDN4 | TGTACCAACTGCCTGGAGGATG | GACACCGGCACTATCACCATAAG | 147 |
| HOTAIR | AGGCCCTGCCTTCTGCCT | TGCTCTCTTACCCCCACGGA | 174 |
| GAPDH | GTGAACCATGAGAAGTATATGACAAC | CATGAGTCCTTCCACGATACC | 215 |
2.4. Statistical Analysis
Statistical analyses were done using GraphPad Prism version 5 software (GraphPad Software, USA) and one-way analysis of variance followed by Newman–Keuls multiple comparison test or student t-test. The P < 0.05 was considered statistically significant. The results were expressed as Mean ± SD.
3. Results
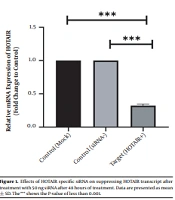
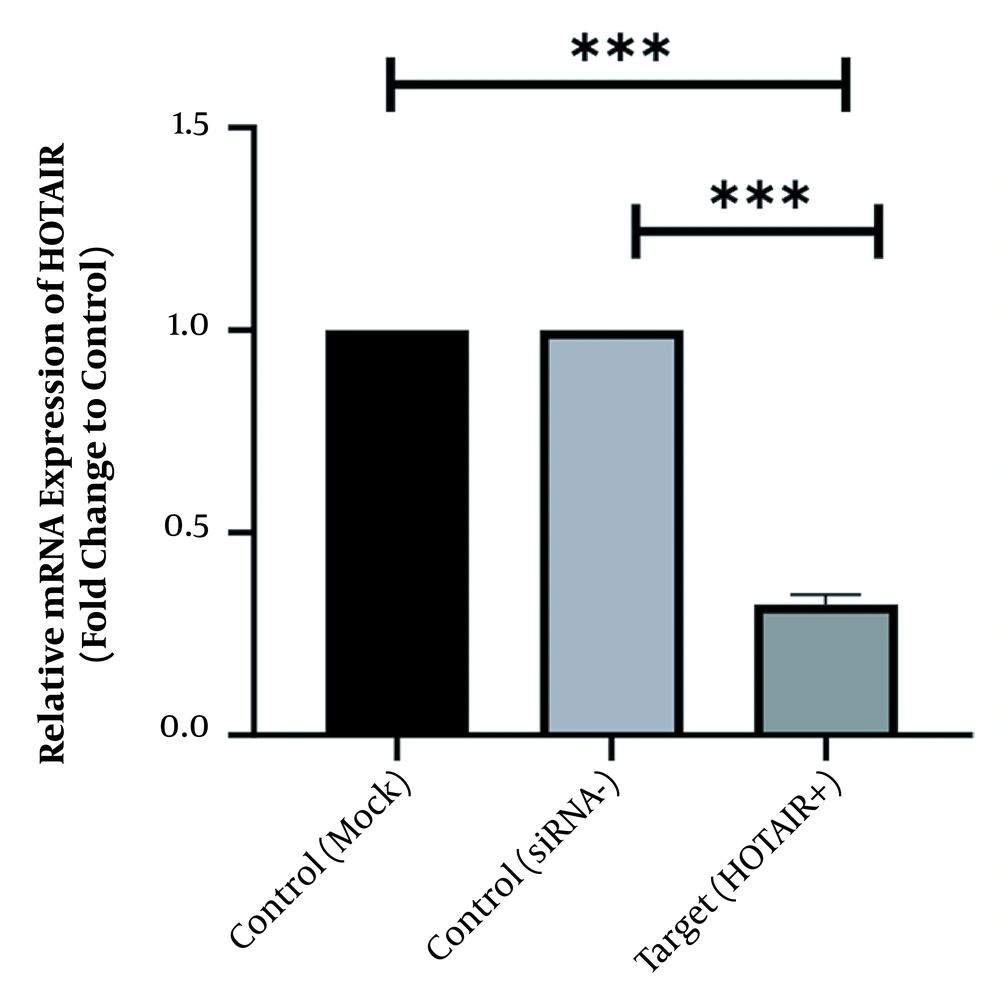
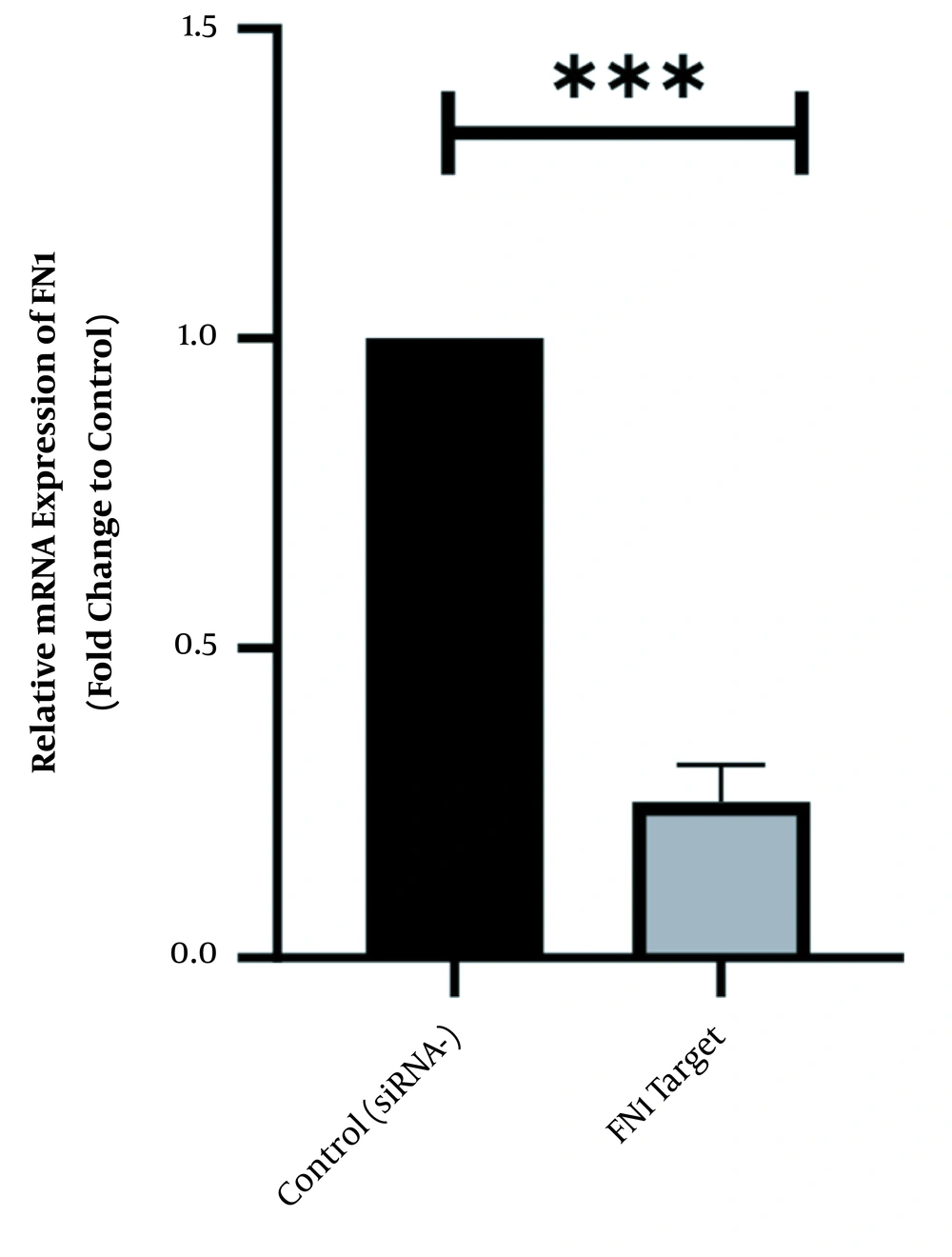
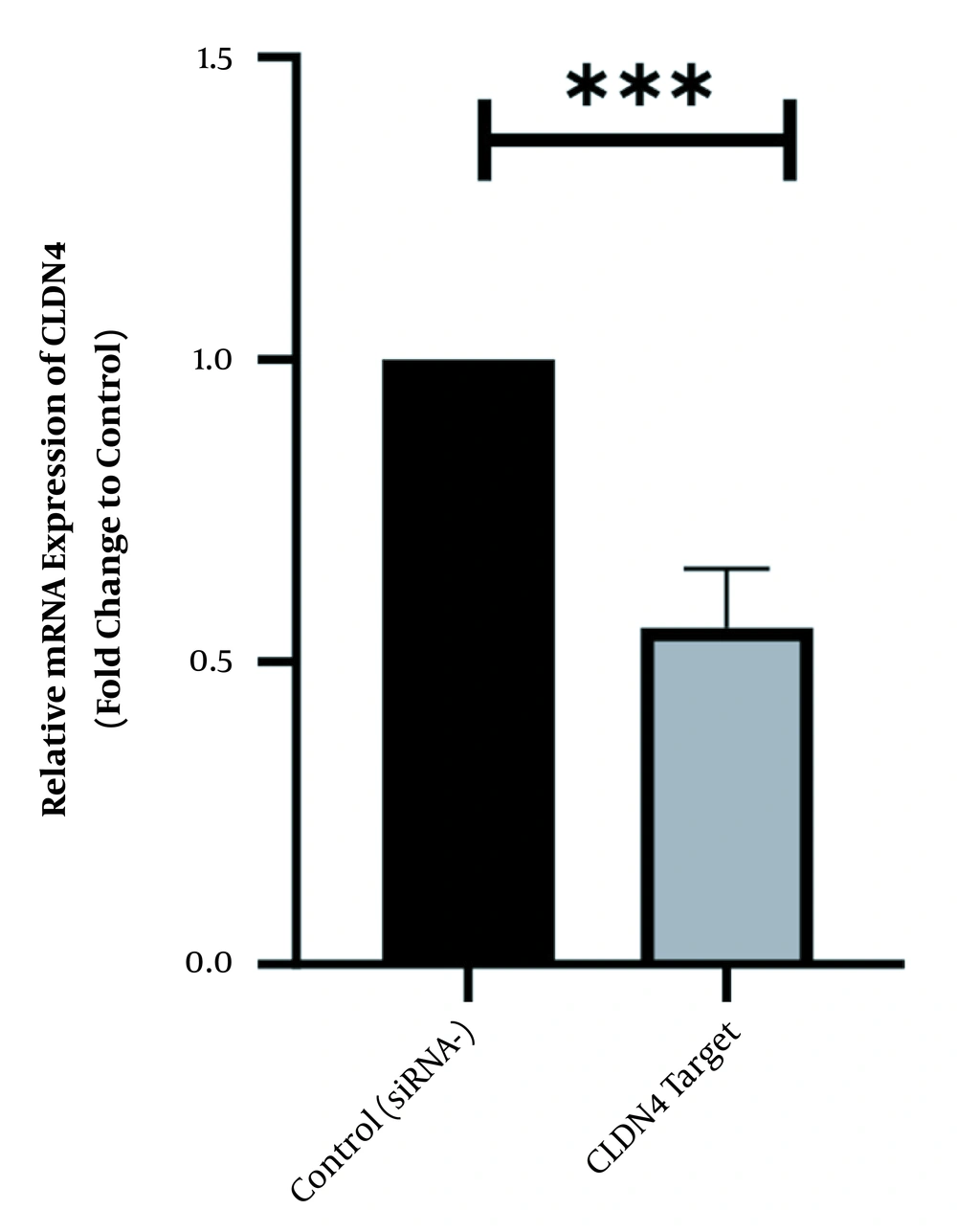
To scan the success of siRNA on silencing of HOTAIR, the expression level of the genes was assessed by real-time PCR. As indicated in Figure 1, in normal culture environments, HOTAIR was robustly expressed in AGS cells. Following 48 h of treatment with 50 ng of HOTAIR - siRNA, the expression level of this lncRNA was significantly diminished in AGS cells. The fold-change was 0.325 ± 0.02379 (P-value < 0.001) (Figure 1). Similarly, the expression levels of FN1 and CLDN4 were also measured by qRT - PCR to address the question of whether the HOTAIR silencing could affect the expression levels of these EMT-associate genes. As illustrated in Figures 2 and 3, before silencing of HOTAIR, both FN1 and CLDN4 were noticeably expressed in AGS cells. However, following HOTAIR specific siRNA post-treatment, the expression levels of FN1 (Figure 2) and CLDN4 (Figure 3) were significantly decreased in comparison to non-treated cells (P < 0.001). The CLDN4 fold-change was 0.5568 ± 0.0971, and the corresponding value for FN1 was 0.252 ± 0.0599. In fact, the amount of CLDN gene was reduced by half, and also FN1 gene was reduced by almost five times following the transfection.
5. Discussion
This is the first study that showed silencing of HOTAIR transcript can affect the expression levels of two important factors of EMT (including fibronectin 1 and claudin-4). The findings showed that both genes were downregulated following the silencing of HOTAIR transcript. As indicated previously, during the EMT, the polarity of epithelial loses, and cells are capable of migrating and metastasis. Cytoskeletal remodeling and resistance to apoptosis are two fundamental consequences of EMT (12). As previously confirmed, HOTAIR can stimulate the EMT and cancer stem cell phenotype in several human cancers (13). HOTAIR silencing significantly reduced the expression levels of EMT players such as fibronectin and claudin in some human cancers, but no data were available about gastric cancer.
Claudin proteins are resided in tight junctions of all epithelia and endothelial cells and regulate the permeability of these cells (14). Claudin-4 (CLDN4) is a 209-amino-acid member of this family, encoding four putative transmembrane fragments (14). The increased expression of cldn4 was firstly observed in pancreatic cancer (15). Consequently, altered expression of this protein was demonstrated in many cancers such as bladder, renal, prostate, endometrial esophageal, and ovarian cancer, in which the expression of CLDN4 has increased (14). Although several claudin isoforms have been structured and studied in mammals, the function of CLDN4 is not well studied; so this variant was considered in this study. Increased expression of CLDN4 was observed in gastric cancer, which was associated with tumor invasion and metastasis (16). This is partly due to the modulatory effect of Claudin-4 on metalloproteinase (MMP) activation and increased expression levels of matrix MMP-2 and MMP-9 (17). Silencing of CLDN4 induced apoptosis in some cancer cells like breast (18). Similarly, siRNA-mediated silencing of this protein efficiently promoted colorectal cancer cell motility (19).
Similarly, fibronectin 1 (FN1) plays an important role in cell adhesion and its interaction with extracellular matrix (20). High expression of fibronectin is a poor prognostic factor in gastric cancer (21). Along with neuropilin, fibronectin 1 regulates EMT in gastric cancer (22). Forced expression of fibronectin 1, inhibits apoptosis and migration in nasopharyngeal carcinoma (23). Fibronectin 1 knockdown increases the mitochondrial-dependent apoptosis in rat mesenchymal cells (24) In conclusion, this research for the first time showed that silencing of HOTAIR can affect the expression levels of two critical elements of EMT, including CLND4 and FN1. However, a long way remains to apply this finding in therapeutic approach and further experiments are needed, including the evaluation of the effect of this silencing on mice models of gastric cancer.