1. Background
Age, as an inevitable and unescapable reality, raises the risk of dementia and Alzheimer's disease (AD). Even when disease symptoms are not overt, aging leads to reduced cognitive capability in different severities among humans. Hippocampus is highly affected by aging and can cause age-related deficits of memory and neuroplasticity. Aging-induced memory deficits might be aggravated by abnormalities in hippocampus functions and structure. Due to the aging, the hippocampus size is reduced, hippocampal neurons spine density and dendritic arbors are suppressed, dentate gyrus (DG) neurogenesis degree is dropped, field excitatory postsynaptic potentials is repressed, long-term potentiation (LTP) is induced, and levels of serum brain-derived neurotrophic factor (BDNF) in the hippocampus decrease (1, 2).
Growing research suggests that environmental factors can contribute to healthy cognitive aging. Three environmental factors affect cognitive reduction and dementia prevention: (1) active social network; (2) leisure activity with cognitive stimulations; and (3) involvement of physical activity. Physical activity is suggested to be the main factor in keeping cognition ability (3).
It is well-known that AD leads to the attenuation of insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), growth and neurotrophic factors (brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF). Thus, growth factors and neurotrophic upregulations can be a good exercise mechanism to save brain from AD. Research shows that AD drastically affects BDNF mRNA and proteins, particularly in hippocampus. It is also reported that reduced levels of BDNF and NGF in the brain result in AD pathology. Besides, lentiviral vectors administration for constitutive expression of BDNF in hippocampus is able to inhibit cellular death, reverse neuronal atrophy, and improve behavioral deficits. Thus AD development is postponed (2).
Nervous system function depends on growth factors and their signaling. Synaptic plasticity and strength in mammalian nervous system is controlled by the family of tropomyosin-receptor-kinase (Trk). As evident in some nervous system diseases, neural developmental disorders might be caused by Trk signaling dysregulation. Tyrosine kinase receptors, in addition to having a part in the nervous system, are involved in the biology of cancer. Besides, in differentiation of the neural precursor stem cells, Trk and neurotrophins as their ligands are included. Trk receptors are growth factor receptors that have tyrosine kinase function and control the strength and plasticity of synapses. As transmembrane proteins, growth factor receptor tyrosine kinases (GFR-TK) participate in cellular growth, differentiation, survival, and apoptosis (4).
Some studies showed the effect of physical exercise on circulating rates of NGF, NT-3, and NT-4 neurotrophins. Contrary to BDNF, the effect of physical exercise on the circulating rates of NGF is not clear yet. A study reported an increase in the concentration of NT-3 and NT-4 biomarkers when an intervention of resistance and combined exercise was applied (5).
In addition to the experiments, treatment with acetylcholinesterase (AChE) enzyme inhibitors is also considered in the literature. These inhibitors ameliorate synaptic acetylcholine central concentrations, which increases cholinergic system neurons interactions within the memory function (6).
Donepezil, which is used for severe AD treatment, is a selective, strong, uncompetitive, and quickly reversible acetylcholinesterase (AChEI) inhibitor with proven efficiency as monotherapy or along with NMDA-agonist (memantine) in some randomized, placebo-managed, double-blinded, short-run clinical experiments and long-run extensions and observations. Donepezil is demonstrated to be a safe and well-tolerated medicine even for multiple co-morbidities under polypharmacy. Obviously, clinical outcomes and treatment results for caregivers and patients are desirable; however, even after 20 years of using donepezil, some challenges are still unresolved as follows: (1) How should responders, as optimal AChE inhibitors mostly in long-term treatment, be selected? (2) Can we recommend increasing the dosage for arriving at a sustained benefit in some patients? (3) What is the best treatment duration, and when is it meaningful to stop the treatment? (7)
2. Objectives
Accordingly, since no study has examined the simultaneous effect of resistance training and use of donepezil, this study aimed to investigate the effect of eight weeks of resistance exercise and donepezil on neurotrophins (BDNF, NT3, and NGF) gene expression and Trk (TrkA and TrkB) receptors in the hippocampus of rats with AD.
3. Methods
The male Wistar rats were purchased from Ahvaz Jundishapur University of Medical Sciences and placed in a room at 22 ± 2°C with a light cycle of 12 hour dark and 12 hour light. The rats had ad libitum water and food access and were divided into Sham (resting and exercising) and STZ (resting Alzheimer and exercising Alzheimer) groups. The former received normal saline while the latter received STZ via intracerebroventricular injection (ICV) in cerebral ventricles.
3.1. Induction of AD Model
In the initial days, the rats were checked for their health and weighed. In the anesthetization step, they received ketamine 10% (100 mg/kg of body weight) and xylazine 2% (10 mg/kg of body weight). After shaving the rats’ skulls, betadine solution was used to disinfect surgery sites. Using stereotaxic device, the coordinates for each rat were precisely measured in terms of weight and anteroposterior distance from bregma to lambda points (Ap: 1.08 mm, ML: ± 2 mm, GV: 4 mm) (8). Then, the calculated points were marked on the skulls’ surface via ink through the axes of the device. Using a manual drill device, the skulls were then perforated. After inserting a Hamilton syringe to a definite depth into skulls, the intracerebroventricular injection (at a rate of 1 μL/min) of a 3 mg/kg body weight dose of STZ synthesized by New Life Science (USA) in a 5 μL serum was conducted. After the injection, the syringe was kept for 5 minutes in its place (9). The wounds were then sutured, and rats were moved to cages until their complete recovery from anesthesia. The animals were then put beside the other rats. The same procedure was repeated for Sham group, except for the fact that they had a physiologic serum injection rather than STZ.
3.2. Passive Avoidance Memory Evaluation
After 14 days of injecting STZ, memory and learning were evaluated using the Passive Avoidance Learning Test, and induction of AD experimental model was confirmed.
3.3. Shuttle box and the training stages:
The Shuttle is rectangular in shape and has two similar Plexiglas-made boxes of dimensions 30 × 20 × 20 cm. At the bottom of each box (5.2 mm in diameter; 8 mm apart), there exist parallel metal rods that can induce electric shock, if needed, in training. The boxes are connected through a 5.6 × 8 cm small door which might be opened or closed, if necessary. The electric shocks was exerted to animals’ paws through the controlling part of the device designed to adjust shock intensity, frequency, and duration (10). Shuttle box training steps were as follows:
- Adaptation: In the first training day, the rats were all exposed to the device for 3 min and were released to mobilize between light and dark parts of the two boxes.
- Training/acquiring: After 24 hours, rats were put in the light section of the device while connecting door was shut. For a better application of electric shock, we wetted the hands and feet of the animals. Half a minute later, we opened the linking door and recorded the time that took the rats to move into the dark part. Any rat refusing to enter the dark box within 300 seconds was excluded from the test. The door was closed after the entrance of the rats into the dark part; after 5 seconds, a 3-second-long 1.5-mA shock was applied to the animals (10). Half a minute later, the rats were exited from the device, and the procedure was then repeated for the remaining rats. The time length for a rat’s movement from the light to the dark box was regarded as latency time at the training initiation (prior to shock exertion), used to examine the rats’ visual and motor capabilities (11).
- Recalling step: The rats’ recalling ability was assessed one day later. Hence, they were placed in the light section for 30 seconds. The door was kept open to record the rats’ entrance time to the dark box. Any rat refusing to enter the dark box within 300 seconds was excluded from the test. Finally, we recorded the time of rats’ presence in the dark box two days after training and shock induction.
3.4. Resistance
The exercising groups received exercise for a total of eight weeks (three alternate days each week). With a weight attached to their tails, the rats were supposed to go over a 26-step ladder. Using their left and right feet and hands equally, the rats picked up the weight 26 times. The introduction and orientation took five days from the exercising groups. On a weekly basis, the animals were weighed precisely. The weight was initially 30% of a rat’s bodyweight, gradually increasing to 150% of the bodyweight in the subsequent seven weeks (30% per week in weeks 2 - 4, and 10% in weeks 5 - 7). In week 8, the weight remained unchanged. To reduce the stress effects, the resting groups were daily handled (12). A Shuttle box was used to study the rats’ memory and learning abilities 24 hours after the final session of training. To remove the last training impacts, 24 hours after the Shuttle test, the rats underwent deep anesthesia and were beheaded (13). After hippocampus extraction and storage at -80°C, the gene expression levels were explored using quantitative reverse transcription polymerase chain reaction (RT-PCR).
3.5. Donepezil Consumption
Two groups of rats, including the Donepezil Alzheimer and Donepezil-Exercise Alzheimer, were fed daily at a dose of 1.5 mg/kg (14). In the Donepezil-Exercise Alzheimer group, in addition to taking donepezil, the samples also performed resistance exercise according to the program.
3.6. Extraction and Reverse Transcription of Total RNA
Tripure RNA Isolation Reagent was utilized to extract total RNA using manufacturer’s manual (the German Roche), and the quality (purity) and concentration were determined by spectrophotometry. Total RNA was reverse transcribed into single stranded complementary DNA (cDNA) via Revert AidTM First Strand cDNA Synthesis Kit (Fermentas, EU).
3.7. Real-time PCR
The iQ5 Multicolor Real Time PCR Detection System was used to conduct real-time PCR analysis. PCR reactions were initiated with 10 minutes of denaturation at 95°C followed by 45 cycles of denaturation at 95°C for 40 seconds, 45 seconds of annealing at 58°C, 40 seconds of extension at 72°C for, and 10 minutes of extension at 72°C. The PCR reactions were all repeated once more. Via 2-DDCT approach, BDNF and other genes’ fold changes or relative expression levels in hippocampus were estimated.
DeltaCT is threshold cycle (CT) differences related to Genes and housekeeping (GAPDH) genes, and DDCt shows DCt resveratrol treated. The size and quality of the PCR products was controlled via electrophoresis on 2% agarose gels.
3.8. Statistical Analysis
Data were expressed as mean±SEM. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test were used to analyze data. P < 0.05 was assumed to be statistically significant
4. Results
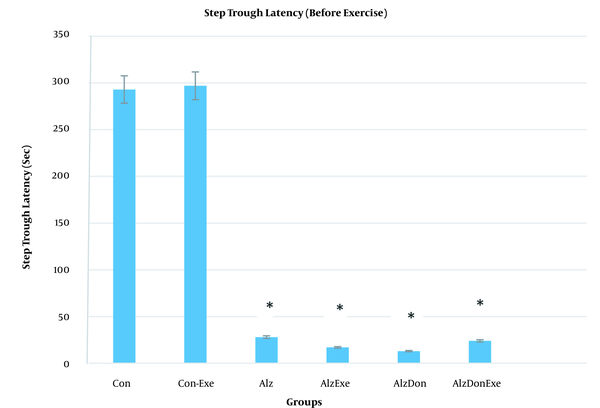
For data analysis, SPSS software (version 22, ANOA, and Tukey’s post hoc test were used (Figure 1).
Results of shuttle box test. Latency time for going into the dark part of Shuttle box prior to training (* significant difference compared to control groups indicates significantly lower latency times prior to resistance exercises, and hence a considerable memory loss for AD group compared to the control group).
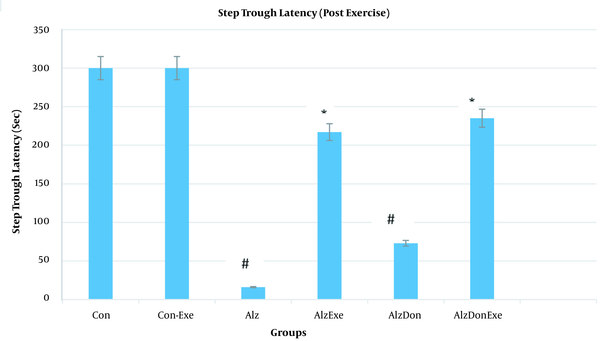
According to Figure 2, the latency time to move into dark box was significantly lower for the resting AD compared to the exercising AD rats. A significant difference was also observed between the resting AD rats and both the resting and exercising control groups. Resultantly, the resistance exercises can postpone the movement into the dark box in exercising AD rats compared to the resting AD group. An improvement was also observed in the memory of the exercising AD group.
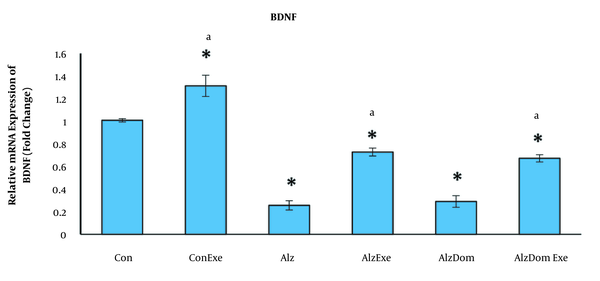
As Figure 3 shows, the level of mRNA expression of BDNF significantly decreased in all studied groups except Con-Exc compared to the control group (P < 0.01 in all cases). This level in Con-Exc was greater than the control group (P < 0.05). The expression level in BDNF mRNA significantly increased in Con, Con-Exc, Alz-Exc, and Alz-Don-Exc groups than in Alz group (P < 0.05). This level was not significant in group Alz-Don compared to Alz group (P < 0.05). This level in Alz-Don-Exc group was significantly higher than in Alz-Dom group (P < 0.05).
Effect of inducing Alzheimer’s disease and exercise on gene expression of BDNF in rats’ hippocampus. This level in all studied groups except Con-Exe decreased compared to the control group (a, significant difference compared to Alzheimer’s group; * significant difference compared to control group).
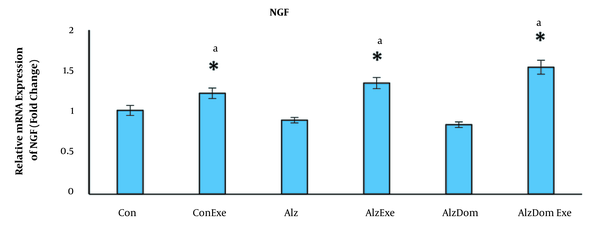
As Figure 4 shows, the level of mRNA expression of NGF significantly increased in all studied groups except Alz and Alz-Don compared to the control group (P < 0.05 in all cases). The mRNA expression levels of NGF in Con, Con-Exe,Alz-Exe, and Alz-Don-Exe groups were higher than Alz group (P < 0.05). This level was not significant in Alz-Don group compared to Alz group (P < 0.05). Also, this level in Alz-Don-Exe group was significantly higher than Alz-Don group (P < 0.05).
Effect of inducing Alzheimer’s disease and exercise on gene expression of NGF in rats’ hippocampus. This level in all studied groups except Alz and Alz-Dom groups increased compared to control group (a, significant difference compared to Alzheimer’s group; * significant difference compared to control group).
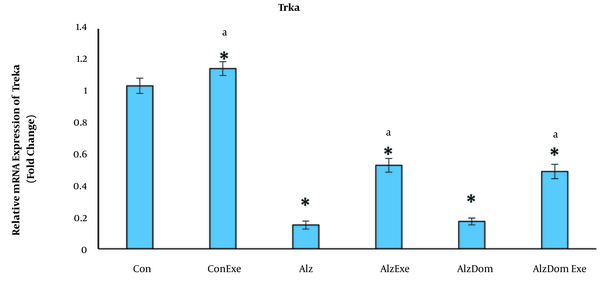
As Figure 5 shows, the level of mRNA expression of TrkA significantly decreased in all studied groups except Con-Exe group compared to control group (P < 0.01 in all cases). The mRNA expression levels of TrkA significantly increased in Con, ConExe, AlzExe and Alz-Don-Exe groups compared to Alz group (P < 0.05). This level was not significant in Alz-Don group compared to Alz group (P < 0.05). Also, this level in Alz-Don-Exc group was significantly higher than in Alz-Don group (P < 0.05).
Effect of inducing Alzheimer’s disease and exercise on gene expression of TrkA in rats’ hippocampus. This level in all studied groups except Con-Exe group decreased compared to control group (a, significant difference compared to Alzheimer’s group; * significant difference compared to control group).
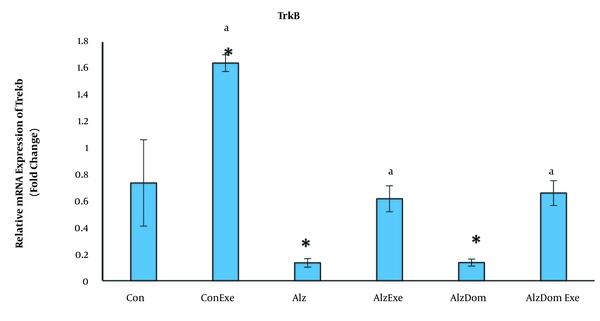
As Figure 6 shows, the level of mRNA expression of TrkB significantly decreased in all studied groups except Con-Exe compared to control group (P < 0.01 in all cases). The mRNA expression level of TrkB significantly increased in Con, Con-Exe, Alz-Exe and Alz-Don-Exe compared to Alz group (P < 0.05). This level was not significant in group Alz-Don compared to Alz group (P < 0.05). Also, this level in Alz-Don-Exe group was significantly higher than in Alz-Don group (P < 0.05).
Effect of inducing Alzheimer’s disease and exercise on gene expression of TrkB in rats’ hippocampus. This level in all studied groups except Con-Exe decreased compared to control group (a, significant difference compared to Alzheimer’s group; * significant difference compared to control group).
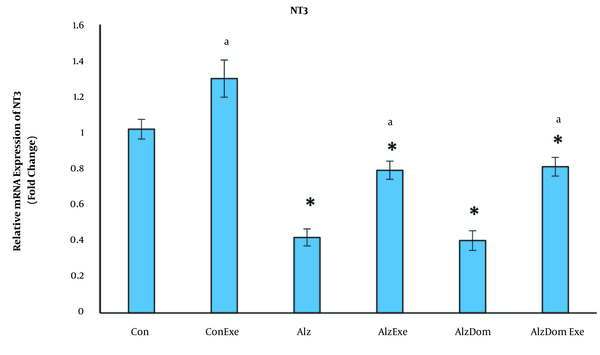
As Figure 7 shows, the level of mRNA expression of NT3 significantly decreased in all studied groups except Con-Exe compared to control group (P < 0.01 in all cases). The mRNA expression level of NT3 significantly increased in Con, Con-Exe, Alz-Exe and Alz-Don-Exe groups compared to Alz group (P < 0.05). This level was not significant in Alz-Don group compared to Alz group (P < 0.05). Also, this level was significantly higher in Alz-Don-Exe group compared to Alz-Don group (P < 0.05).
Effect of inducing Alzheimer’s disease and exercise on gene expression of NT3 in rats’ hippocampus. This level in all studied groups except Con-Exe decreased compared to control group (a, significant difference compared to Alzheimer’s group; * significant difference compared to control group).
5. Discussion
The results of present study showed that the expression of all the genes studied in all groups except the Donpezil group was significantly different from the Alzheimer's group. While the results of the present study regarding the effect of exercise, especially resistance training on neurotrophins gene and their receptors expression, are consistent with those reported by Cullen (3), Wang and Holsinger (15), and Lippi et al. (5), they are inconsistent with the results of Dinoff et al. (16), Walsh et al. (17), and Dinoff et al. (18). The difference in results can be due to the environmental measurement of the research variables; in our research, the variables were measured directly in the hippocampus.
The results of our research indicated that the use of donepezil alone for eight weeks did not have a significant effect on the expression of the studied genes. These results are inconsistent with those reported by Leyhe et al. (6) and Jelic and Darreh-Shori (7) possibly due to different doses of the drug and the duration of use. In the above-mentioned research, donepezil was used at a dose of 10 mg/kg for 1.5 to 2 years, while in the present study the dosage was 1.5 mg/kg for eight weeks.
The results of the study showed that resistance training and taking donepezil at the same time significantly increased the expression of the studied genes. No study was found on the effect of exercise, especially resistance training, and concomitant use of donepezil on the expression of neurotrophins gene in the hippocampus.
Our results showed that donepezil consumption with the dose used in this study did not significantly increase the expression of the genes studied, but the use of donepezil with eight weeks of resistance training significantly increased the genes studied. This mismatch is possibly due to the effect of exercise on increasing cerebral circulation, neurogenesis, and increasing brain signaling in terms of gene expression.
Regarding the effect of resistance training and donepezil consumption on learning, our results also showed that performing eight weeks of resistance training and concomitant use of donepezil significantly increased learning. These results are consistent with the results of Jiangbo and Liyun (19). Accordingly, it can be said that resistance training and donepezil use can increase learning by increasing neurogenesis, increasing the expression of neurotrophins and their receptors, increasing cerebral blood flow, reducing the activity of acetylcholinesterase, and reducing the neuronal apoptosis.
Physical exercise can increase cognitive ability. Among different mechanisms involved in the interdependence between physical activity and brain health, much attention is recently given to neurotrophins receptor signaling pathway, which supports the growth and differentiation of new synapses, axons and dendrites and increases the synaptic plasticity and the survival of neurons. The present study showed that physical exercise and circulating brain-derived neurotrophic factor levels are positively interconnected. Besides, the change in this molecule after exercises is accompanied by the betterment of neurocognitive functioning. More definite results are still needed for other neurotrophins, like nerve growth factor, neurotrophin-3, and neurotrophin-4 (5).
It is evident that AD results in the attenuation of neurotrophic and growth factors (BDNF, IGF-1, VEGF, and NGF). Thus, up-regulation of neurotrophic and growth factors can form a viable exercise strategy for brain protection against AD. According to clinical and basic studies, BDNF mRNA and proteins (e.g., proBDNF) are negatively affected by AD, particularly in hippocampus. It is also reported that reduced levels of BDNF and NGF in the brain result in AD pathology. Besides, lentiviral vectors administration for constitutive expression of BDNF in hippocampus is able to inhibit cellular death, reverse neuronal atrophy, and improve behavioral deficits, thus AD development is postponed. In addition to increasing neurotrophic factors generation, exercise as a physiologic mechanism can induce BDNF expression in hippocampus to minimize the AD-induced functional impairments of hippocampus. The improved BDNF signaling through exercise can mitigate learning and memory impairments in AD by increasing neurogenesis and LTP expression, modulating dendrites shape, and reversing Aβ-caused neurotoxicity (2).
BDNF concentrations are more heavily and robustly influenced by aerobic exercise rather than resistance exercise. A meta-analysis of 12 trials of randomized control through resistance intervention showed that the level of BDNF in blood did not change significantly after exercise protocols that varied in type, duration, frequency, and time (18). Walsh et al. (2016) observed that resistance exercise did not change basal BDNF concentrations (17). Blood samples were taken in 20 min increments for a total of 2 h, with time 0 at the start of exercise and under resting condition. The BDNF blood concentrations increased transiently through exercise, but receded within 2 h; and the rising and falling responses of BDNF concentrations to the exercise before and after the 8-week exercise protocol were similar (3).
Considering the time and cost limitations, we could not use more drug doses in a longer time period to increase the results efficiency. While increasing neurotrophic factors generation, exercise as a physiologic mechanism can also induce BDNF expression in hippocampus to minimize the AD-induced functional impairments of hippocampus. The improved BDNF signaling through exercise can mitigate learning and memory impairments in AD by increasing neurogenesis and LTP expression, modulating dendrites shape, and reversing Aβ-caused neurotoxicity.