1. Context
Gliomas are the most common type of primary brain tumors, accounting for about 30-80 percent of all brain tumors and malignant tumors, respectively (1). World Health Organization (WHO) has classified tumors into four grades, based on malignancy, with grade I tumors having the least malignant behavior and grade IV tumors the most (1-3). Glioblastoma, previously known as glioblastoma multiform (GBM), belongs to grade four, which is the most malignant form. It can also be the most feared type of primary brain tumor not only for its aggressiveness and high levels of proliferation but also for its effects on the quality of life (1, 4, 5). This tumor, on average, usually develops at the age of 64 with a 14.8 percent of survival rate at 2 years (1). Therefore, among common gliomas, glioblastoma has the least survival rate (1). There is also a molecular classification for glioblastoma that comprises of four subtypes, based on different gene alterations (2). These subtypes include mesenchymal, proneural, and neural glioblastomas (Table 1) (2). Because of the cellular complexity of glioblastoma and the atypical-multiform appearance of its cells (that vary in size and shape), the term 'spongioblastoma multiform' was introduced by Bailey and Cushing in 1926 (2, 4-8). Today, the term 'spongioblastoma multiform' is replaced with 'glioblastoma' and the word 'multiform' which represented the complexity and heterogeneous histologic appearance of cells was abandoned in the 2007 WHO classification of tumors of the CNS, and now it is simply called ' glioblastoma' (5, 6). Despite different methods available for diagnosis, treatment, and management of patients with glioblastoma, prognostic factors such as age, gender, the extent of tumor resection, and neurological status play an important role in the overall outcome of a patient (1, 3, 9). While various factors can be used to classify glioblastoma, generally, it is characterized into primary and secondary glioblastomas (Table 2) (3, 9, 10), which was first presented by Hans Joachim Scherer (1906 - 1945), the German neuropathologist (3, 8). Primary glioblastoma, also known as wild-type IDH (wild-type isocitrate dehydrogenase) glioblastoma, is the most common type of glioblastomas, accounting for more than 90 percent of cases (incidence rate: 2.578 per 100000 people per year according to World Standard Population) and the time between its emergence and development is so short that no specific clinical or histological evidence of a less malignant precursor can be observed, which is the main reason that it is named as 'de Novo' glioblastoma (3, 5, 10). The clinical history of patients with primary glioblastoma is usually less than three months, and it is more prevalent in men (3). The median age of patients with this type of tumor is 62 years; however, it also occurs in children and young adults (1, 3). The two most frequent gene alterations in primary glioblastoma are LOH10q (70%) and EGFR amplification (36%) (2, 3, 10). Secondary glioblastoma, also known as IDH mutated glioblastoma, develops slowly from a low-grade glioma (WHO grade II diffuse astrocytoma or WHO grade III anaplastic astrocytoma) and therefore it has a long clinical history (1, 3, 4, 10). In contrast to primary glioblastoma, it usually develops in younger patients with a mean age of 45 (incidence rate: 0.167 per 100000 people per year) and appears more frequently in women, with a median survival rate of 7.8 months (3). The two most frequent gene alterations in secondary glioblastoma are TP53 mutation (65%) and LOH10q (63%) (3).
| Glioblastoma Subtypes | Alterations |
|---|---|
| Classic | Chromosome 7 amplification, chromosome 10 loss, EGFR amplification |
| Mesenchymal | Loss of NF1, presence of CD44 and MERTYK markers of epithelial to mesenchymal transition |
| Proneural | IDH1 point mutations and PDGFRA alterations |
| Neural | Expression of NEFL, GABRA1, SYT1, SLC12A5 as neural markers |
Molecular Classification of Glioblastoma
| Items | Primary Glioblastoma | Secondary Glioblastoma |
|---|---|---|
| Mean age | 62 | 45 |
| Sex | Common in Men | Common in Women |
| Most frequent gene alteration (s) | LOH10q and EGFR amplification | TP53 mutation and LOH10q |
| Clinical history | Short | Long |
| Mean survival rate (mo) | 4.7 | 7.8 |
General Comparison Between Primary and Secondary Glioblastoma
2. Evidence Acquisition
In writing this article the National Center for Biotechnology Information (NCBI) was the primary resource, followed by the Google Scholar database. Keywords such as 'glioma', 'glioblastoma', 'PTEN', 'LOH', 'TP53', 'IDH1', and 'glioblastoma novel treatments' were used in the process of writing this article. The titles and abstracts were reviewed for choosing the best and appropriate articles.
3. Results
3.1. EGFR/PTEN/Akt/mTOR Pathway
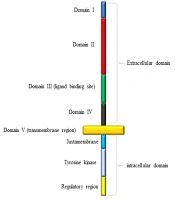
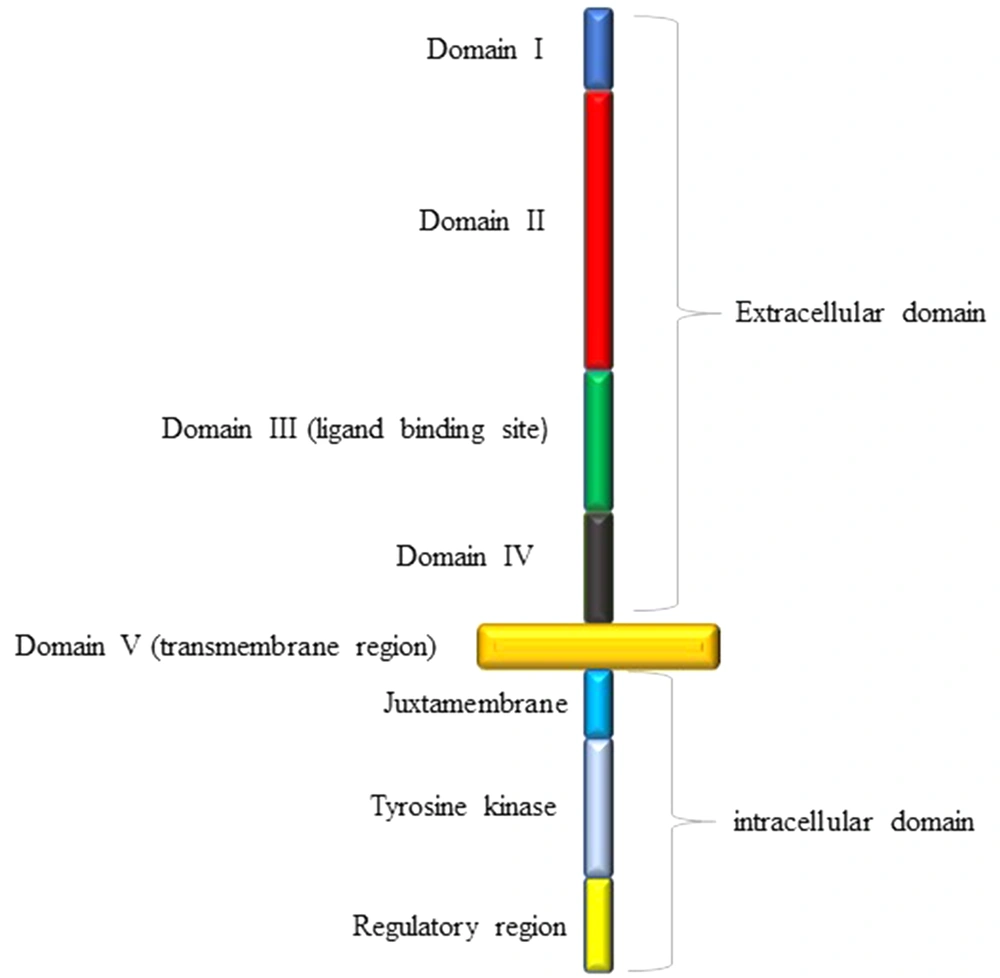
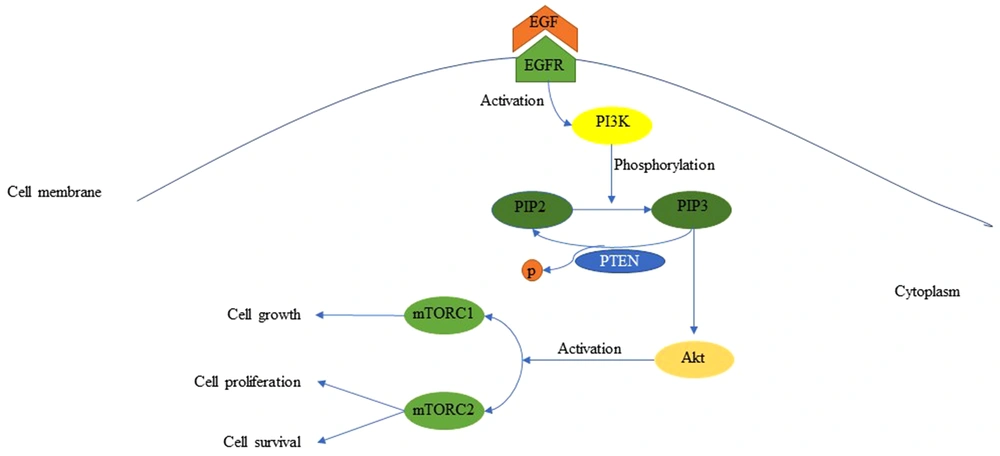
The tyrosine kinase epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein that stimulates cell survival, mobility, growth, and proliferation (3, 11-13). This receptor is made of a single polypeptide chain with three domains (Figure 1) (14); the extracellular domain with four regions, including the ligand-binding site (14), the intracellular domain consisting of the regulatory region, tyrosine kinase, and juxtamembrane, and lastly the transmembrane domain (14). EGFR becomes activated when appropriate ligands bind to its extracellular domain (3, 10, 14). Ligands, which can bind to EGFR, are epidermal growth factor (EGF), transforming growth factor-α (TGF-α), amphiregulin (AR), heparin-binding EGF-like growth factor (HB-EGF), and betacellulin (BTC) (14). The attachment of EGF to EGFR induces the activation of phosphatidylinositol 3-kinase (PI3K) that in turn phosphorylates phosphatidylinositol 4, 5-bisphosphate (PIP2), and turns it into phosphatidylinositol 3,4,5-triphosphate (PIP3) (Figure 2) (3, 10-12, 15). The activated PIP3 yields Akt, also known as protein kinase B (PKB), which promotes the activation of mTOR1 and mTOR2 complexes (3, 10, 11). The mTOR complex 1 can cause cellular growth by decreasing and increasing catabolic and anabolic reactions of proteins and lipids, respectively, while mTOR complex 2 can lead to cellular proliferation and cell survival (3, 10, 11). The existence of PTEN is necessary due to its capability of inhibiting the PI3K signaling (3, 10, 11, 16). PTEN is a multifunctional tumor suppressor and a genome stabilizer that has important effects on many biological processes, including cell growth, cell proliferation, cell survival, cell invasion, and cell migration (3, 8). Loss of PTEN results in the accumulation of PKB due to the loss of PI3K blockage (8, 16). Although PTEN mutation almost exclusively occurs in primary glioblastoma, EGFR amplification accompanied by EGFRvIII, the most common deletion mutation in the extracellular domain of EGFR in human cancer, stays on the top of the list of primary glioblastoma genetic alterations (3, 14). EGFR has different modifications, but the most crucial ones are EGFR amplification, overexpression, and EGFRvIII deletion (2, 3, 6, 11, 14). EGFRvIII rarely occurs without the co-existence of EGFR amplification (3, 11). This mutation can enhance cell proliferation through PI3K/AKT pathway by down-regulation of p27. Furthermore, EGFRvIII deletion mutation is usually the result of intragenic rearrangements; however, alternative splicing of mRNA can also lead to this (3, 14).
EGFR/PTEN/Akt/mTOR pathway is the key signaling pathway capable of developing primary glioblastoma, and the EGFR gene seems to be an auspicious therapeutic target since its amplification and overexpression have been detected in 40 - 60 percent of cases dealing with glioblastoma, respectively, and it is rarely detectable in normal brain tissue (3, 11).
3.2. P16INK4/RB1 Pathway
The retinoblastoma (RB) gene, called RB1 (located on chromosome 13q14.2), is known to be an important tumorigenesis factor, especially in glioblastoma (3, 10, 17, 18). Its promoter methylation is more frequent in secondary glioblastoma, and there is a correlation between RB1 gene promoter methylation and loss of RB1 expression, which may lead to loss of RB1 function and cancer (3, 10, 19). This loss of expression is known to be a prognostic factor in many human neoplasms, including glioblastoma, and is associated with higher grades of malignancy (17). Retinoblastoma protein (pRB) has the ability to control the G1 to S phase progression (3, 10, 18). Phosphorylated pRB is not associated with transcription factor E2F. Therefore, it results in the G1 to S phase transition (3, 10, 12). E2F is a transcription factor divided into three activators (E2F1-E2F3), three canonical repressors (E2F4-E2F6), and two atypical repressors (E2F7, E2F8), and all of these are involved in cell cycle regulation (18, 20). This transcription factor is so important and can be used as a prognostic marker, apart from a cancer marker (18). Phosphorylated pRB cannot prevent G1 to S phase progression; however, hypophosphorylated pRB binds to E2F and prevents G1 to S phase transition (3, 10, 12). The phosphorylation of retinoblastoma protein is accomplished by Cyclin D/CDK4 complex (3, 10, 12). While phosphorylated pRB results in the release of E2F and G1 to S phase progression, P16INK4, a protein that slows down the progression of G1 to S phase, inhibits Cyclin D/CDK4 complex. Therefore, it prevents G1 to S phase progression and acts as a tumor suppressor gene (3, 6, 10, 12, 21).
3.3. TP53/MDM2/P14ARF Pathway
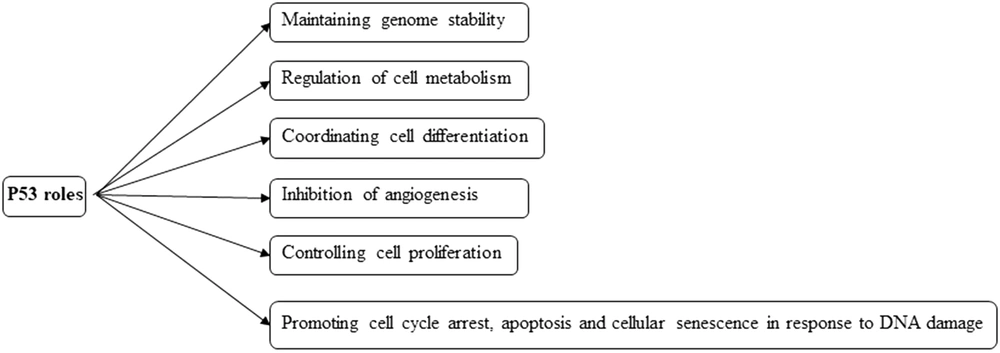
TP53 gene is located on chromosome 17p13.1 and encodes a 393-aminoacid protein called p53, which has a crucial role in coordinating many cellular responses (Figure 3). Therefore, this protein has a momentous role in preserving cellular homeostasis and is rightly called the ''Guardian of the Genome'' (3, 10, 12, 22). Under normal cell conditions, p53 functions are controlled by MDM2 (mouse double minute 2 homolog) and MDM4 (MDMX), which are the two negative regulators of p53 through ubiquitination and degradation (12, 22). According to TCGA (The Cancer Genome Atlas), the P53/MDM2/P14ARF pathway is deregulated in 84% of glioblastomas, mainly in secondary glioblastoma (3, 10, 22). P53 protein is frequently activated in response to abnormal cellular conditions such as DNA damage, hypoxia, genotoxicity, and oncogenic activities and induces tumor suppressor activity by altering the expression of genes, which are involved in processes such as apoptosis, cell cycle arrest, and cellular senescence (3, 22). The inactivated status of p53 protein is associated with more aggressive, more proliferative, and less apoptotic glioblastoma (22). In this pathway, the CDKN2A locus is usually deleted or inactivated (12). Due to the importance of the genes, which can be expressed from this locus, such as p16INK4 and P14ARF, the presence of this locus can lead to the inhibition of MDM2, one of the TP53 inhibitors (10, 12, 22). P14ARF inhibits MDM2 (12). There is a negative feedback loop between p53 and MDM2 (3, 22). MDM2 transcription is originated by p53, which is the target of MDM2 for inhibition (3, 22). Since p53 mutation has a high level of expression in secondary glioblastoma, it can be administered as an important target for medicine therapies (3, 22). A prominent strategy is to restore wild-type p53 by inhibiting the MDM2/p53 complex using inhibitors such as Nutlins (22), which may lead to wild-type p53 degradation prevention (22).
3.4. IDH1
Tumors that are associated with IDH1 mutation seem to have a better prognosis (5). The presence of IDH1 mutation is known to be a highly important molecular difference between primary and secondary glioblastoma (22). This mutation is a heterozygous missense mutation (12). It is present in nearly 75 percent of secondary glioblastomas, while it can barely be recognized in primary glioblastomas (22). IDH1 gene is located on chromosome 2q33 (10) and provides instructions for synthesizing an enzyme called isocitrate dehydrogenase, which is found in both cytoplasm and peroxisome, to catalyze the production of α-ketoglutarate from isocitrate in tricarboxylic acid (TCA) cycle (3, 10, 12, 23, 24). While wild-type IDH1 acts in this way, the mutant IDH1 generates D-2-hydroxyglutarate from α-ketoglutarate (12, 24, 25). The accumulation of D-2-hydroxyglutarate inhibits the normal activity of α-ketoglutarate dependent enzymes such as histone demethylase and TET (ten-eleven translocation) family of 5-methylcytosine hydroxylase (1, 25). Despite this drawback, it is still a valuable target for personalized anti-cancer therapy (25).
3.5. LoH
Loss of heterozygosity 10q is the most frequent (70%) gene alteration in primary glioblastoma and the second most common gene alteration (63%) in secondary glioblastoma (3, 10). A common deletion in chromosome 10q25-qter occurs in both of these grade IV glioma subtypes (3, 10). LoH 10p is almost exclusively present in primary glioblastoma, and LoH 22q and 19q are more frequent in secondary glioblastoma (3, 10). LoH 10q is usually co-expressed with one or two more gene alterations resulting in the development of both grade IV glioma subtypes (3). LoH 1p and 13q can also be detected in both primary and secondary glioblastomas but at low frequencies (3, 10). The most frequent loci deleted in LoH are 10p14-15, 10q23-24, and 10q25-qter (3, 10). The 10q25-qter loci have several important tumor suppressor genes such as MXI1 at 10q25.1, h-neu at 10q25.1, and abLIM or LIMAB1 at 10q25. They are also associated with the histological transition from anaplastic astrocytoma to grade IV glioma phenotypes, their deletion in both glioblastoma subtypes, and their role in pathogenicity in both grade IV glioma subtypes (if deleted or not present), making it an important candidate requiring further investigation (3, 26).
3.6. Protein Expression Profile
Different protein expression in primary and secondary glioblastomas reflects the variety of gene alterations in these tumors (3, 10). EGFR, tenascin-X precursor, centrosome associated protein 350, and enolase 1 are the four proteins that are expressed uniquely in primary glioblastoma (3, 10). In secondary glioblastoma, however, proteins such as ERCC6, DUOX2, HNRPA3, WNT-11 protein precursor, ADAMTS-9, and cadherin-related tumor suppressor homolog precursor are typically expressed (3, 10). Furthermore, ASCL1 is overexpressed in 88% of secondary glioblastomas. This overexpression is accompanied by the blockage of Notch signaling (3, 10).
3.7. Glioblastoma Stem Cell Therapy
Since glioblastoma stem cells (GSCs) can cause invasive tumor growth, their signaling pathway is known to be very important in cancer stem cell therapy (27). New therapeutic targets can be recognized based on the GSC signaling pathways (27). Osteopontin (OPN) is a matricellular protein that can serve as a central node for connecting the Wnt pathway, cell cycle, and focal adhesion (27). Since the inhibition of OPN can result in a prolonged survival rate and the formation of neurospheres are OPN-dependent, this protein's inhibition can be a good therapeutic strategy in stem cell therapy for eradicating GSCs while dealing with glioblastoma (27). Glioblastoma stem cells are usually resistant to chemotherapy due to the presence of the MDR1 gene plus P-glycoprotein (P-gp) (27). While dealing with glioblastoma and using chemotherapy for a long time, an increase in DNA-dependent protein kinase (DNA-PK) and P-gp level can be seen (27). DNA-dependent protein kinase (DNA-PK) can level up the expression of P-glycoprotein. PI3K/Akt pathway can be used for the probable increase of MDR1 level via DNA-PK. Therefore, targeting DNA-PK may be effective in eliminating the GSC (27).
3.8. Symptoms, Treatments, and Clinical Managements
Headache is the most frequent symptom of glioblastoma, which is present in approximately 50 percent of diagnosed patients (4). However, this symptom is not specific, but there are some features that can differentiate a benign headache from a headache caused by a tumor (4). These features include persistent headache with nausea and vomiting, headache with accompanying cognitive symptoms, a headache that may awaken the patient even in its mild form, and unilateral pain that does not change side and is limited to the side in which the tumor is located (4, 28). Other presentations of glioblastoma include visual deficiency, aphasia, and personality changes (4, 28). Patients' age and stage of the disease are major factors that should be considered when deciding about the treatment strategy (2). Currently, surgery is the common first-line treatment of glioblastoma (4, 29). It is crucial to resect as much of the tumor as possible by surgery to reduce the tumor load, which not only offers relief from its mass effect and symptoms but also provides valuable tissue samples for histological and molecular diagnosis (5). In addition, radiotherapy has also been proven to be an appropriate treatment (5). In addition to these two options, temozolomide (TMZ), which is a DNA-alkylating agent designed to cross the blood-brain barrier to achieve therapeutic goals due to its lipophilic nature, is also promising (5, 25, 30). TMZ was discovered in 1970 and approved by FDA in 2005 (2). It adds a methyl group at the O6, N3, and N7 sites of DNA residues and causes cell cycle arrest at G2 to M phase transition stage, which can ultimately cause apoptosis (5, 30, 31). O6-methylguanine-DNA methyltransferase (MGMT) is an enzyme that repairs the O6 and N7 sites of guanine alkylated by TMZ (2, 31). TMZ works when the MGMT promoter is methylated (2, 31). This incidence results in the insertion of a thymine base instead of a cytosine base during the upcoming DNA replication, and therefore it can cause cell death (31). Using TMZ as the only therapeutic regimen will give the advantage of an oral treatment without the need of a daily radiotherapy (5). In addition to the monotherapy with TMZ, temozolomide and TTFields adjuvant, also known as tumor treating fields or alternative electric field therapy, works better than TMZ alone (5, 32). TTFields application not only can reduce the invasion of cancer cells but also hinders the mobility and migration of such cells (32). Corticosteroids and bevacizumab are frequently used for treating glioblastoma as well (4, 5). In addition, corticosteroids can decrease cerebral edema with a side-effect of suppressing the immune system (5). Bevacizumab targets VEGF (vascular endothelial growth factor), a mediator of tumor neovascularization, to stop and eliminate cancer angiogenesis (2). Bevacizumab can be a good option for recurrent glioblastoma treatment as it can suppress the supply of nutrients required for tumors, which can otherwise be gained through angiogenesis (4).
4. Conclusions
Glioblastoma is a malignant and hardly-survived tumor that uses different cellular pathways and manifests various genetic alterations for its development. LOH10q and TP53 mutation were the most frequent gene alterations recognized in primary and secondary glioblastoma, respectively. Due to recent advances in understanding the cellular and molecular complexity of this tumor, several promising medical approaches have been included in treatment strategies, including the usage of nutlins, TMZ, and TTFields. Although this tumor is the toughest among gliomas, there is hope for new and better therapeutic options, which may ease the diagnosis and treatment process of this invasive tumor.