1. Background
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by chronic abdominal pain, changes in normal bowel movements, and altered bowel behaviors (constipation and/or diarrhea) in the absence of structural and biochemical disorders (1, 2). The median prevalence of IBS ranges from 10 to 15% around the globe (3, 4). Epidemiological studies have shown the prevalence of IBS between 5% and 10% in Asian countries, while it is relatively high (about 10 to 18%) in western countries (5, 6). The prevalence of this disease has been reported about 6% and 6.2% in Iran and Kerman Province (southeast of Iran), respectively (7, 8).
The precise etiology of the disease is unclear (9), but several studies have suggested that some factors such as mental stress (10), autonomic nervous system disorder (11), gastrointestinal movement disorders (12), gut microbiota changes (13), and genetic susceptibility (14) may be involved in the development of IBS. According to previous reports, about 10% of patients develop IBS after an intestinal infection (15).
Currently, the diagnosis of IBS is based on the Rome IV criteria (16). Persistent symptoms of low-grade inflammation of the intestinal mucosa have been reported in IBS patients (17). In recent years, there has been a greater association between gut microbiota disorder and the pathophysiology of IBS, and it has been shown that intestinal flora (intestinal microbiota) in IBS patients is significantly different from that in healthy individuals (18).
The association between viruses and various diseases with unknown etiology, including multiple sclerosis (MS), lupus, rheumatoid arthritis (RA), Guillain-Barre syndrome, scleroderma, and IBD, has been evaluated, and it has been reported as one of the probable etiologies (19-22). In a study conducted in Iran, an increase in the level of viral pattern recognition receptors (PRRs) in IBS patients was observed compared to the control group, and the possible role of viruses as one of the possible causes of IBS was reported (23). Rotaviruses, adenoviruses, noroviruses, and astroviruses are the most common pathologic viruses that can cause gastroenteritis in the gastrointestinal tract (24-26). Among these, adenoviruses are one of the most important causes of acute gastroenteritis among children (27, 28). Most of the human adenoviruses in the intestinal epithelial cells cause subclinical infections after ingestion and entry into the gastrointestinal tract (29). Since children have a weaker immune system compared with adults, the immune system has less preventive control over the spread of viruses; thus, the risk of transmission of the disease increases (30). Given the role of adenoviruses in diarrhea and gastroenteritis in children and neonates, as well as the reports of changes in viral recognition receptors in IBS patients, it is suggested that individuals who develop more severe or frequent adenoviruses infections are more likely to develop IBS in adulthood. Active adenovirus infection is another factor that may be related to the etiology of IBS. If it is found that viruses are the cause of IBS, vaccination against these viruses may prevent the disease in adulthood. Understanding the etiology of IBS can also help clinicians predict and design new and effective methods to control and treat the disease, thereby improving the patients’ quality of life.
2. Objectives
To the best of our knowledge, several studies have examined the association between various types of viruses and IBS, but no study has examined the role of adenovirus infection in IBS. In this study, we aimed to investigate the association of adenoviruses as one of the most common gastrointestinal viruses with IBS. It should be noted that although adenoviruses 40 and 41 are involved in gastrointestinal diseases, in this study, we sought to investigate the presence and amount of adenoviruses (from each side) in patients and controls.
3. Methods
3.1. Study Population
Forty patients with symptoms suggestive of IBS according to the Rome IV criteria were identified and diagnosed by an experienced gastroenterologist from the Gastroenterology Clinic of the Mehregan Hospital (Kerman, Iran) from March 2017 to December 2018. This group was included patients with various types of IBS (IBS-D, IBS-C, and IBS-M). Written informed consent was obtained from all the participants. This case-control study was conducted under the guidelines set by the Ethics Committee of Kerman University of Medical Sciences (code: IR.KMU.AH.REC.1396.2066). The study was performed according to the Declaration of Helsinki.
The control group included 40 confirmed healthy individuals referred to Seyed-o-Shohada Hospital (Kerman, Iran). Control subjects were queried about the Rome IV criteria and included in the study if they did not fulfill the criteria. They had no symptoms and no history of IBS, inflammatory bowel disease (IBD), celiac disease, colon cancer, immunodeficiency diseases, and food hypersensitivity. Some relevant tests, such as endoscopy, were performed to exclude other diseases such as IBD and malignancy. The case and control groups were matched based on age and sex. Before sampling, informed consent and questionnaires were obtained from the participants. Data on age, sex, body mass index (BMI), academic education, smoking status, alcohol intake, clinical symptoms, infection, taking non-steroidal anti-inflammatory medications, antibiotics, and family history of IBS or gastrointestinal diseases were obtained by completing the questionnaire. Blood and stool samples of participants were collected. After serum separation, serum and stool samples were immediately stored at -40°C until use.
3.2. Enzyme-Linked Immunosorbent Assay
The adenovirus serology of the subjects (adenovirus IgG and adenovirus IgM) was analyzed by enzyme-linked immunosorbent assay (ELISA) using commercial kits. The sensitivity and specificity of the adenovirus IgG ELISA kit (Cat no. IB79203, IBL, Germany) have been estimated to be > 99% and > 99%, respectively. All the procedures were done as instructed by the manufacturer. The optical density (OD) of 1.00 IU/mL was considered for both negative control and cut-off calibrator in the IgG test. The presence or absence of anti-adenovirus IgG antibodies is defined by comparing the sample absorbance with the cut-off calibrator absorbance. Samples with lower OD from the 0.8 IU/mL (cut-off) were considered negative results for anti-adenovirus IgG antibodies, while samples with OD higher than the cut-off calibrator were considered positive for anti-adenovirus IgG antibodies. Samples with absorbance values varying within ± 10% of the cut-off calibrator were considered suspected results (gray zone). The measurement of anti-adenovirus IgG antibodies was made quantitatively based on 1, 10, 35, and 150 IU/mL concentrations of calibrators.
Adenovirus IgM antibodies were detected by the Human Anti-adenovirus IgG ELISA Kit (Cat no. IB79202, IBL, Germany). The sensitivity and specificity of the adenovirus IgM test have been estimated to be > 99% and 98%, respectively. For IgM antibodies, OD calculations of samples were performed. The presence or absence of anti-adenovirus IgM antibodies was defined by comparing the sample absorbance to the absorbance of the cut-off control. Samples with lower OD compared to cut-off control OD were considered negative results for anti-adenovirus IgM antibodies, while samples with higher OD compared to cut-off control OD were considered positive results for anti-adenovirus IgM antibodies. Samples with absorbance values ranging within ± 10% of the cut-off control were considered suspected results and re-tested for confirmation (gray zone). An OD of 0.8 IU/mL was considered for both negative control and cut-off calibrator in the IgM test. The measurement of anti-adenovirus IgM antibodies was made quantitatively based on 1, 10, 40, and 150 IU/mL concentrations of calibrators.
3.3. Adenovirus DNA Extraction
One gram of stool samples was transferred to a 15-mL Falcon tube; then, 5 mL of normal saline treated with 5 µL of 10% Sodium dodecyl sulfate (SDS) was added to the tube. After centrifugation at 2000 rpm, 2 mL of the supernatant was transferred to a 2-mL microtube and centrifuged at 5000 rpm. Afterward, 1 mL of the supernatant was transferred to the new microtube, and 20 µL of Proteinase K (Yekta Tajhiz Azma, Iran) was added to each microtube and incubated at 37°C for 2 hours. Then, 220 µL of the saturated NaCl (5 M) was added to the tube and vortex and centrifuged at 7000 rpm for 15 minutes. The supernatant was transferred to the new microtube, and 250 µL of absolute ethanol was added to the tube (31, 32). After vortexing and centrifugation at 14000 rpm for 15 minutes, the supernatant was discarded, and 250 µL of Tris-EDTA was added to each microtube. Then, 250 µL of phenol-chloroform-isoamyl alcohol (25:24:1) was added, and after vortexing for 1 minute, it was centrifuged at 7000 rpm for 10 minutes. At this stage, 2 phases were formed. The supernatant was removed and transferred to the new microtube, and 250 μL of absolute ethanol was added again. After centrifugation at 14000 rpm, the supernatant was discarded, and the sedimented DNA was dissolved in 20 µL of distilled water (33). In this extraction protocol, the stool samples mixed with confirmed adenovirus and sterile distilled water were used as positive and negative controls, respectively. The quality and quantity of the extracted DNA were evaluated by the NanoDrop ND2000c Spectrophotometer system (Thermo Scientific, USA), and DNA concentration was set to 50 ng/μL.
3.4. Real-time Polymerase Chain Reaction
The presence of adenovirus DNA and viral load were analyzed with real-time polymerase chain reaction (PCR). Primers were designed for the adenovirus genome using Primer version 3 and checked by Primer-BLAST and OligoAnalyzer version 3.1. Human growth hormone (hGH) gene primers were also used for internal control in real-time PCR. Primer sequences are shown in Table 1. The positive control of adenovirus DNA and a no template control (NTC) were included in each PCR run. Reactions with melting curve analysis were performed using a RealQ Plus 2x Master Mix Green High Rox (Amplicon, Denmark) in an ABI Step One Real-time PCR instrument (Applied Biosystems, USA). A real-time PCR amplification reaction was performed in a 20-μL reaction mixture containing 10 μL of master mix, 0.5 μL of each forward and reverse primers (10 pM), 5 μL of nuclease-free sterile distilled water, and a 4-μL aliquot of extracted DNA. Thermal cycling consisted of initial denaturation and enzyme activation at 95°C for 15 minutes, followed by 45 cycles of denaturation at 95°C for 15 seconds, annealing at 60°C for 15 seconds, extension at 72°C for 15 seconds, and final melting curve generation steps from 65 to 95°C with 0.3°C/min increments. Each sample was run in duplicate; average adenovirus cycle thresholds (Cts) per run were recorded, and samples were considered positive if both replicates had Ct values ≤ 40.
| Gene | Primer Sequence (5´→3´) | Length (bp) | Annealing Temp (ºC) | Product Size (bp) |
|---|---|---|---|---|
| Adenovirus | F: GCCACGGTGGGGTTTCTAAACTT | 23 | 60 | 127 |
| R: GCCCCAGTGGTCTTACATGCACATC | 25 | |||
| hGH | F: TCACGGATTTCTGTGTTTC | 19 | 60 | 140 |
| R: TCACGGATTTCTGTTGTGTTTC | 22 |
Abbreviations: hGH, human growth hormone; temp, temperature; bp, base pair.
3.5. PCR Product Size Validation
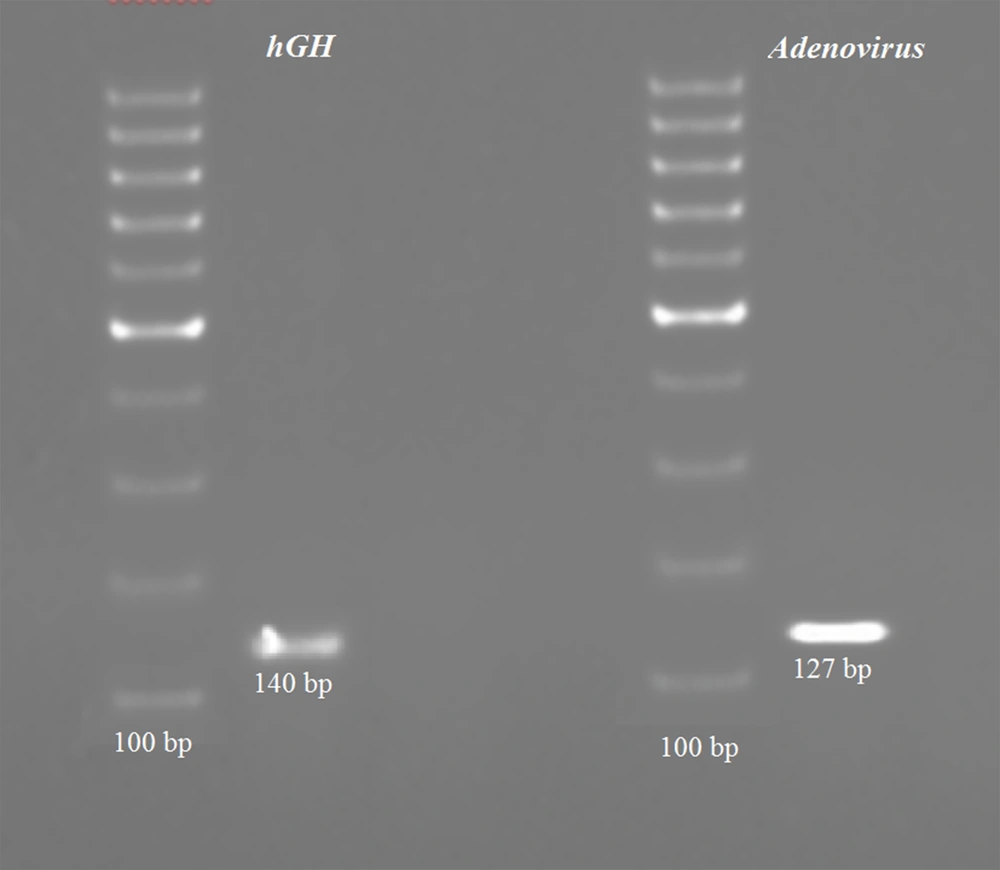
Adenovirus and hGH gene amplification was performed using PCR, and the products were validated by the presence of 140 and 127 base pairs (bp) fragments on 2% agarose gel electrophoresis, respectively. A representative gel image of amplified PCR products of hGH and adenovirus genes is shown in Figure 1.
3.6. Data Analysis
The continuous data were analyzed by an independent Student t-test. Data normality was analyzed by Kolmogorov-Smirnov and Shapiro-Wilk tests. Categorical variable data analysis was performed using the χ2 or Fisher exact test. Multivariable logistic regression was used to quantify the association between adenovirus infection and IBS risk, estimating odds ratios (ORs) and their 95% CIs. All P-values were 2-sided, and P-values less than 0.05 were considered statistically significant. Data analysis was performed using SPSS 22.0 (SPSS Inc, Chicago, IL, USA).
4. Results
The cases of IBS were predominantly men (65%) and had a mean age of 34.35 ± 11.66 years, with a low history of IBS among their first-degree relatives (10%) and low physical activity (65%). The controls were, on average, 2 years older (36.64 ± 11.90), with a higher family history of IBS (17.5%) and a higher academic education rate (60%). As shown in Table 2, the demographics were similar in both groups. No statistical differences were observed in age, sex, academic education, smoking status, alcohol intake, physical activity, and family history in both groups, but the BMI was lower in IBS cases (P = 0.009). The clinical symptoms related to IBS showed significant differences in both groups except for nausea, vomiting, and diarrhea-constipation situation (Table 2).
| Demographic and Clinical Characteristics | Case (N = 40) | Control (N = 40) | P-Value |
|---|---|---|---|
| Age (y) | 34.35 ± 11.66 | 36.64 ± 11.90 | 0.389 |
| Sex | 0.329 | ||
| Male | 26 (65.0) | 30 (75.0) | |
| Female | 14 (35.0) | 10 (25.0) | |
| Academic education | 16 (40.0) | 24 (60.0) | 0.117 |
| BMI (kg/m2) | 22.57 ± 1.73 | 23.75 ± 2.17 | 0.009 |
| Current smoking | 7 (17.5) | 5 (12.5) | 0.531 |
| Alcohol intake | 11 (27.5) | 9 (22.5) | 0.606 |
| Physical activity | 0.338 | ||
| Low | 26 (65.0) | 20 (50.0) | |
| Moderate | 10 (25.0) | 16 (40.0) | |
| High | 4 (10.0) | 4 (10.0) | |
| Family history of IBS | 4 (10.0) | 7 (17.5) | 0.336 |
| Clinical symptoms | |||
| Abdominal pain | 37 (92.5) | 1 (2.5) | < 0.001 |
| Bloating | 31 (77.5) | 2 (5.0) | < 0.001 |
| Constipation | 9 (22.5) | 2 (5.0) | < 0.001 |
| Diarrhoea | 26 (65.0) | 0 (0) | < 0.001 |
| Nausea | 3 (7.5) | 0 (0) | 0.077 |
| Vomiting | 4 (10.0) | 1 (2.5) | 0.359 |
| Diarrhoea-constipation | 3 (7.5) | 0 (0) | 0.077 |
| Depression | 18 (45.0) | 7 (17.5) | 0.015 |
| Anxiety | 14 (35.0) | 5 (17.5) | 0.035 |
| Clinical findings | |||
| Anti-adenovirus IgG (IU/mL) | 180.25 ± 17.97 | 177.46 ± 18.11 | 0.910 |
| Anti-adenovirus IgM (IU/mL) | 1.60 ± 0.64 | 1.97 ± 0.10 | 0.764 |
| Adenovirus viral load | 0.174 ± 0.02 | 0.169 ± 0.02 | 0.267 |
| Adenovirus DNA+ | 26 (65.0) | 21 (52.5) | 0.364 |
a Values are expressed as mean ± SD or No. (%). P-values less than 0.05 were considered statistically significant.
Overall, adenovirus seropositivity was 37 (92.5%) in IBS cases and 31 (77.5%) in control subjects. The results of the ELISA test showed no significant difference in the concentration of anti-adenovirus IgM antibodies between the case and control groups (P = 0.764). Also, the differences in IgG antibody concentration were not significant between both case and control groups (P = 0.910). Most of the IBS and control subjects were seropositive for IgG and IgM antibodies, and the differences in the antibodies concentration were not statistically significant. Also, the comparison of the presence of the adenovirus genome in stool samples of patients and controls was not statistically significant (P = 0.364). Most participants in the case and control groups were positive for the presence of adenovirus DNA in stool samples. IBS patients had somewhat lower Ct values (ie, higher viral loads) compared with healthy controls (0.174 ± 0.02 vs 0.169 ± 0.02; P = 0.267; Table 2), but this difference was not statistically significant. Therefore, viral loads showed no significant difference between the IBS and control groups (P = 0.267).
5. Discussion
IBS is a common gastrointestinal disorder with a high prevalence and heavy financial burden on the economy. Studies have shown that 12% of people suffer from IBS worldwide (34). The precise etiology of IBS is still unclear; thus, further studies can be useful in identifying the etiology of the disease. Recently, several studies have reported the role of viruses in developing IBS. In the study by Marshall et al. (35), the effective role of noroviruses in the development of IBS symptoms after acute viral gastroenteritis caused by food was reported. In 2012, Zanini et al. (36) reported the development of IBS after an epidemic of viral gastroenteritis caused by drinking water. The mechanism by which viral gastroenteritis predisposes humans to IBS is unknown, but many studies have suggested that some specific viruses may cause changes in the intrinsic immune PRRs in IBS patients (37). Viruses can also affect intestinal flora by altering PRRs (37). This idea about IBD has previously been proposed by Wang et al. (22). In their study, the role of virus-bacteria interactions in the intestine and changes in the normal intestinal flora as one of the possible causes of IBD were reported.
In the present study, it was revealed that almost all of the participants were qualitatively positive for the anti-adenovirus IgM antibody. Also, the difference in the concentration of IgM antibodies was not significant in the groups (P = 0.764). IgM antibodies are produced in the first encounter with an antigen; thus, IgM is a group of antibodies whose concentration increases in acute infections (38). The positivity of all participants for IgM may be due to the widespread presence of adenoviruses in the environment and the frequent exposure of individuals to these viruses. However, the results obtained in this regard are inconsistent with the results of some studies. Erles et al. (39) investigated IgM antibodies against Adeno-associated Virus-2 (AAV-2) and found that 13% of adults had this antibody. Thiele et al. (40) also reported that 15% of adults had this antibody. As most studies in this field are outdated, new studies with larger sample sizes are recommended. Since acute adenovirus infection in the patient and control groups was not significant, it is suggested that in IBS patients, acute adenovirus infection did not develop simultaneously; however, further studies are recommended to investigate the role of other viruses.
Also, all participants were positive for anti-IgG antibodies, which is consistent with previous reports (39, 40). In this study, the concentration of this antibody in the patient group was not significantly different from that in the control group (P = 0.910), indicating that the association between chronic adenovirus infection and the incidence of IBS seems unlikely if the presence of IgG is considered a chronicity sign of the disease and not a person’s immunity.
At a significance level of 0.05, there was no significant difference in the concentration of the adenovirus genome in the stool samples of patients and controls (P = 0.958). In other words, all participants were positive for the adenovirus genome. This finding confirms the results of the concentration of serum IgM in participants. Therefore, it seems that there is no significant relationship between active infection and adenoviruses and the incidence of IBS; however, this claim cannot be substantially confirmed because this study was performed on the blood and stool samples of IBS patients; thus, further studies are recommended to investigate the patients’ gastrointestinal discharge and biopsy because many adenoviruses are amplified in and infect the epithelial cells of the gastrointestinal tract. Therefore, these viruses can lead to IBS by developing an infection that cannot be detected by fecal PCR. Also, it is worth mentioning that this relationship cannot be generalized to other viruses, especially those in the gastrointestinal tract. According to previous studies, 10% of IBS patients develop the disease following an intestinal infection (15). Previously, the association of some viruses with gastrointestinal diseases of unknown sources has been investigated, and a significant relationship between Epstein-Barr virus (EBV) and IBD has been reported (41).
5.1. Conclusions
Our study cannot establish a significant relationship between adenovirus infection and IBS; thus, we suggest real-time PCR or immunohistochemistry of intestinal biopsy for adenoviruses that may be moving to layers close to the intestinal nerves and causing IBS symptoms. Adenoviruses seem unlikely to play a significant role in the incidence of the disease. However, further studies are needed to confirm our results or investigate the role of other viruses in the digestive tract.
5.2. Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, or the printer/publishers are responsible for the results/findings and content of this article.