1. Background
Microorganisms are the most important causative agents of common oral diseases such as caries and periodontal diseases. In this type of disease, the microbial flora of the mouth is altered by various factors such as environmental factors and antibiotic use. Microorganisms in the oral cavity can communicate by producing metabolic products or exchanging molecular signals. The possibility of microbial biofilm formation increases due to the intercellular connections of microorganisms (1).
Biofilm formation is one of the most critical factors in tooth decay. A microbial biofilm is a community of microbes stacked together in an extracellular matrix of polysaccharides. Microbial biofilm is one of the most important causative agents for many oral infectious diseases, including tooth decay, gingivitis, periodontitis, periapical periodontitis, and peri-implantitis (1, 2). Controlling and inhibiting the formation of oral biofilms impose high costs on patients (3). The biofilm formation cycle includes bacterial binding, biofilm growth/maturation, and biofilm dispersion. Each biofilm formation stage can be inhibited, and solutions that can disrupt any step of the biofilm cycle provide a possible way to control biofilm formation (4).
Pathogens in the form of planktonic cells are always easier to control than pathogens in mature biofilms and do not require higher concentrations of antimicrobial agents (2, 5). However, the biofilm matrix reduces drug access and prevents the drug from penetrating deep layers (6, 7). This feature of biofilm has caused even the most effective methods of controlling oral diseases to fail due to the increased resistance of biofilms to antibiotics and other antimicrobial agents (8, 9).
Various bacterial species have been identified to form relatively stable biofilms on tooth enamel, including Streptococcus ovalis, Streptococcus mutans, Streptococcus mitis, Actinomyces, Lactobacillus, Staphylococcus aureus, and Enterococcus faecalis (10). Among various bacteria, S. mutans plays a vital role in forming cariogenic biofilms (11), where they can rapidly use food sucrose for efficient EPS synthesis and acid production (2).
There are new strategies for inhibiting and eradicating oral biofilms, including the use of nanomaterials, quaternary ammonium salts, small molecules, arginine, and natural products. One of the main biological goals in preventing tooth decay is to reduce the bacterial load that can form biofilms. Common treatments such as fluoride or chlorhexidine (CHX) are used for oral diseases. Fluoride is a compound with a local effect on the tooth surface/plaque, exhibiting anti-caries activity and preventing acid production by S. mutans (12). However, fluoride is not a robust antimicrobial agent. In most treatments, the combination of fluoride with antimicrobial agents such as xylitol and CHX has been recommended by some guidelines for preventing tooth decay, especially in high-risk individuals (11, 13).
As mentioned, CHX gluconate is the most commonly used disinfectant. However, CHX has side effects such as a burning sensation or changing the taste (14). It also has cytotoxicity. Improper use of antibiotics and various chemical compounds increases antibiotic resistance in pathogens and causes side effects in the body and secondary infections. Therefore, there is a need to look for alternatives or modifiers in antimicrobial therapy. To reduce the aforementioned treatments' adverse effects, it is necessary to study natural products with antimicrobial activities inhibiting the formation or removing microbial biofilms (15). Due to their availability and relatively low cost, natural products with antimicrobial activity can be helpful to replace or modulate the adverse effects of antibiotics and common chemical compounds used in the treatment (16).
Natural products include miswak, chitosan, and propolis (11, 17). Over the years, propolis has been studied as a natural and non-toxic compound with antimicrobial and anti-decay properties (18, 19). Propolis is a resinous substance collected from various plants by bees (20). The biological activity of propolis is related to its polyphenols (flavonoids and phenolic compounds), terpenoids, and cinnamic acid (2, 21). Flavonoids have potentially beneficial effects as antimicrobial agents. Various studies have shown the sound antimicrobial effects of polyphenols on different bacterial strains such as Escherichia coli, S. aureus, S. mutans, Streptococcus sanguinis, and Actinomycosis viscosus (22, 23). Propolis has many clinical applications, e.g., to improve gastrointestinal disorders, bacterial and candidal infections, and oral diseases (24). Propolis efficacy has been studied for oral infections, caries prevention, periodontal treatment, and candida-associated denture stomatitis. Besides, propolis's antimicrobial and anti-biofilm activity have been studied in vitro (21, 25, 26). Propolis has also been reported to inhibit the growth of S. mutans and its ability to adhere to tooth surfaces. Propolis also reduced the accumulation of human dental plaque and its insoluble external polysaccharide content (25, 26). The ethanolic extract of propolis inhibits the growth of S. mutans, and its inhibitory effect is comparable to that of CHX against lactobacilli, P. intermedia, P. gingivalis, A. israelii, and Candida Albicans (20, 27, 28). However, its effects against oral pathogens have been compared with other oral antiseptics in a limited number of laboratory studies (29, 30).
2. Objectives
This study aimed to investigate the synergistic effect of CHX and propolis aqueous extract on biofilm inhibition and eradication of common oral biofilm-producing pathogens, namely S. mutans and S. aureus. The minimum biofilm inhibitory concentration (MBIC) and minimum biofilm eradication concentration (MBEC) of CHX and propolis against these microorganisms were determined. Then, the synergistic effects of these two compounds on biofilm inhibition of the studied bacteria were evaluated in vitro using the checkerboard method.
3. Methods
3.1. Preparation and Culture of Bacterial Strains
This study was performed in October 2021. For the preparation of ATCC 35668 S. mutans and S. aureus PTCC 1112 standard strains, samples of lyophilized bacteria were prepared from the cell bank of the Pasteur Institute of Iran. Each strain was cultured under standard conditions. To activate the physiological needs for the growth of strains, they were first inoculated in Tryptic Soy Broth (TSB) (Merck, Germany) medium. Streptococcus mutans was incubated under anaerobic conditions (5 - 10% CO2) and S. aureus under aerobic conditions for 24 h at 37°C. After initial incubation and confirming the growth of the strains (based on the medium turbidity), they were heated in the culture medium containing the grown strains to the size of an inoculated fluid loop on the Tryptic Soy Agar (TSA) (Merck, Germany) medium under the mentioned incubation conditions. This was done to confirm the purity of the strains.
3.2. Determination of MBIC by Broth Microdilution Method
We cultured the studied bacteria on a TSA medium to determine the MBIC of propolis aqueous extract (Roodin food industries, Iran) and CHX 0.2% (Shahre Daru, Iran). After overnight incubation at 37°C, bacterial colonies were removed by loop and inoculated in 5 mL of TSB medium overnight at 37°C. Each row of a 96-well microplate (SPL, Korea) was filled with 100 μL of TSB medium. For each bacterium, propolis aqueous extract was diluted to a 30 mg/mL concentration in a dilution series of 14.64 - 7500 µg/mL in each row of the plate (10 wells). Then, a 0.5 McFarland suspension with a volume of 100 µL was added to each well. Wells 11 and 12 were considered bacterial growth control (positive control) and culture medium sterility control (negative control), respectively. Microplates were used to determine the MBIC after 48 hours of incubation at 37°C. The mentioned steps were performed for CHX at concentrations of 2 mg/mL in the 0.97 - 500 µg/mL dilution series for both bacteria. To detect the minimum concentration inhibiting biofilm formation, we drained the wells' contents and washed the wells three times with physiological serum. In the next step, the 2, 3, 5-tri-phenyl-tetrazolium chloride (TTC) (Merck, Germany) staining method was used to observe the biofilms (27). In this method, the plates were dried under sterile conditions for 15 min, and then 170 mL of TSB culture medium and 30 mL of 2% TTC solution were added to each well. The microtiter plates were placed in the dark at 37°C and 130 rpm for 5 - 6 hours. Next, the contents of the wells were transferred to another microplate, and the absorbance was read at 490 nm by an ELISA Plate Reader (Biotek-epoch). Finally, the MBIC of CHX and propolis aqueous extract was determined for the mentioned bacteria after each experiment was repeated three times. Also, the percentages of biofilm formation inhibition by CHX and propolis aqueous extract for S. aureus and S. mutans were calculated using the following formulas (28).
Percentage of biofilm = (Absorption of the intended well - Absorption of negative control well) / (Absorption of control well - Absorption of negative control well) × 100
Percentage of inhibition of biofilm formation = 100 - Percentage of biofilm
3.3. Determination of MBEC by Broth Microdilution Method
In this test, it was necessary to form a biofilm of both bacteria in separate microplates before treatment with different dilutions of CHX and propolis aqueous extract. Therefore, 100 µL of TSB medium with 100 µL of microbial suspension with a concentration similar to the 0.5 McFarland standard was inoculated into each well, except for the negative control. Microtiter plates were incubated at 37°C for 48 h. The contents of the wells were then drained, and the wells were washed three times with 37°C salines. In the next step, 100 µL of TSB medium and 100 µL of CHX or propolis solution were added to each well of the microplate specific for each bacterium. The desired dilutions were prepared in sterile tubes. Then, 250 μL of TSB culture medium and 250 μL of solutions were combined in sterile tubes, and dilutions were made in 10 tubes. Finally, 200 μL was added to each well, and the microtiter plates were incubated at 37°C for 48 h. After this period, the contents of the wells were removed and washed three to five times with physiological serum, and after drying, the wells were stained with TTC. After six hours, absorbance was measured at 490 nm. Each experiment was repeated three times.
3.4. Investigation of the Synergistic Effect of CHX and Propolis by the Checkerboard Method
The combined effect of CHX and propolis aqueous extract on inhibiting biofilm formation of the studied bacteria was determined by the checkerboard dilution technique. Thus, each horizontal row of microplates was assigned to a density of CHX and each vertical row to a density of propolis aqueous extract. The desired dilutions of CHX and propolis aqueous extract were prepared separately using a TSB culture medium as diluent. The obtained MBIC was used as the dilution center for each solution, and the dilutions of 1.2, 1.4, 1.8, 1.16, 2, 4, and 8 times the desired MBIC were used as the default of the synergy test. Then, 50 µL of each dilution was added to each microtiter plate well. Finally, 100 µL bacterial suspensions were added to the wells. Each plate contained positive and negative controls. The prepared microtiter plates were incubated at 37°C for 48 h and then used for MBIC determination. After incubating, the wells were drained, washed, and stained with TTC dye, and after six hours, the absorbance was measured at 490 nm.
Finally, the fractional minimum inhibitory concentration (FMBIC) of each solution and ∑FMBIC were calculated for the studied cases to determine the presence or absence of a synergism effect (27).
The value of FMBIC determines the effect of the simultaneous use of two agents, as follows:
∑FMBIC ≤ 0.5 indicates the presence of a synergistic effect.
MFMBIC > 4 indicates the presence of an antagonistic effect.
0.5 > ∑FMBIC > 4 indicates indifference between CHX and propolis.
3.5. Evaluation of the Synergy Effect on the Eradication of Biofilm Formation of the Studied Bacteria by the Checkerboard Method
Similar to the study of the synergistic effect of MBIC, different concentrations of CHX and propolis aqueous extract were used from the concentrations determined in MBEC alone. The MBEC obtained for CHX, and propolis aqueous extract was used as the dilution center, and dilutions of 1.2, 1.4, 1.8, 1.16, 2, 4, and 8 times the desired MBEC were used as the default of the synergy test. Following biofilm formation in the wells and draining and washing the wells, the selected dilutions of CHX and propolis aqueous extract were prepared separately using a TSB culture medium as a diluent. Then, 50 µL of each solution and 100 µL of TSB medium were added to each well. The prepared microtiter plates were incubated at 37°C for 48 h and used to determine MBEC.
The presence or absence of a synergism effect was determined by calculating the fractional minimum biofilm eradicating concentration (FMBEC) of CHX and propolis aqueous extract and ∑FMBEC for the studied cases, according to the previous section.
3.6. Statistical Analysis
Data analysis was performed using GraphPad Prism version 5 (GraphPad software, USA) and Minitab version 17.1. (USA) software to study MBIC, MBEC, and synergistic effects. Data analysis for MBIC and MBEC and graphs related to percentage of inhibition, eradication of bacterial biofilms, and synergistic effects of propolis and chlorhexidine were performed descriptively. The significant difference was determined in the synergistic effect of propolis and CHX on MBIC, after confirming the normality of the data, by a two-way analysis of variance (ANOVA) with pair-wise comparisons using Tukey’s method. A P value of < 0.05 was considered statistically significant.
4. Results
4.1. Results of MBIC of Propolis Aqueous Extract and CHX 0.2%
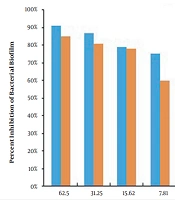
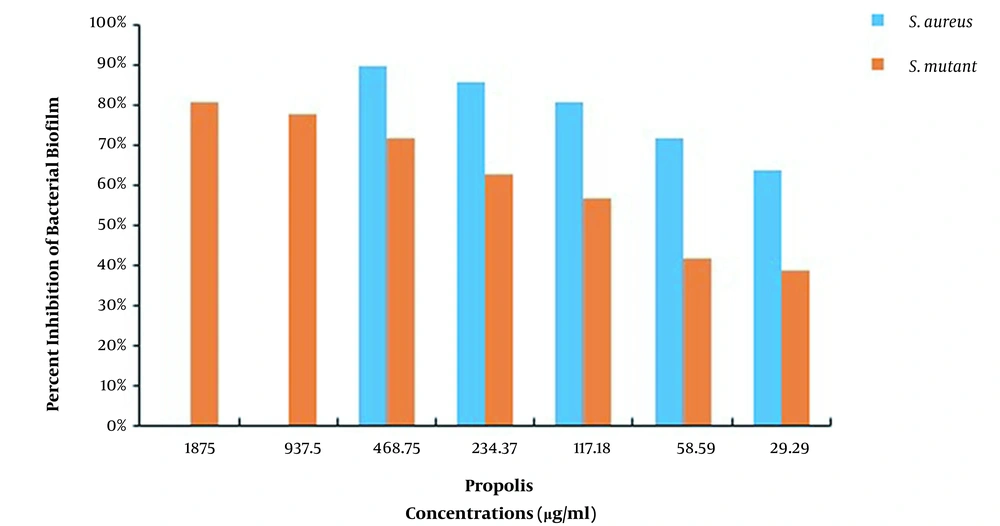
The MBIC results of CHX and propolis aqueous extract for S. aureus and S. mutans are shown in Table 1. The results showed that the MBIC of chlorhexidine for both S. aureus and S. mutans was 125 μg/mL, while the MBIC of propolis aqueous extract was 937.5 and 3750 μg/mL, respectively. Also, after measuring the absorbance at 490 nm, the percentage of biofilm formation inhibition in each case was calculated, as reported in Figures 1 and 2.
| Antibacterial Agent | Range of Concentration (µg/mL) | MBIC (µg/mL) | |
|---|---|---|---|
| Streptococcus mutans | Staphylococcus aureus | ||
| CHX | 0.97 - 500 | 125 | 125 |
| Propolis | 14.64 - 7500 | 3750 | 937.5 |
4.2. MBEC Results of Propolis Aqueous Extract and CHX 0.2%
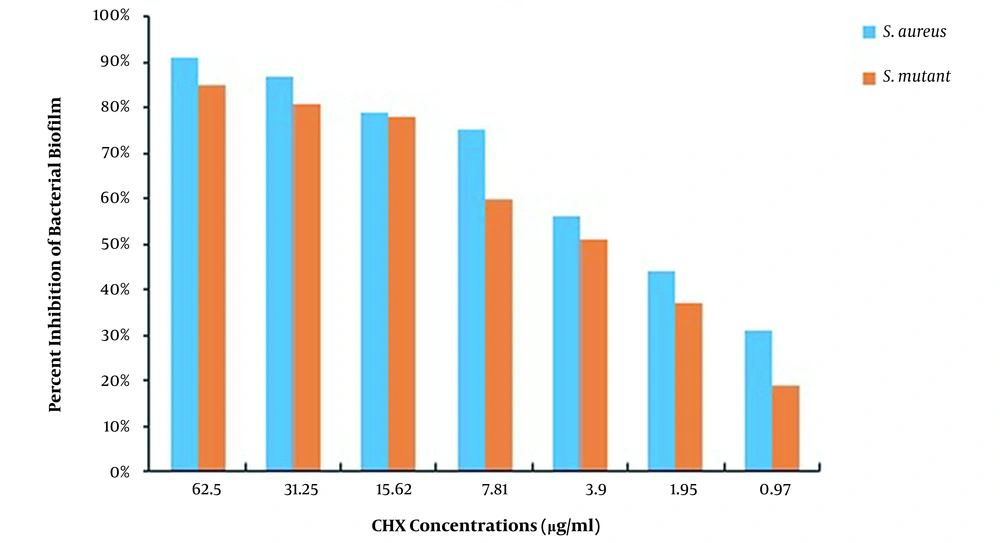
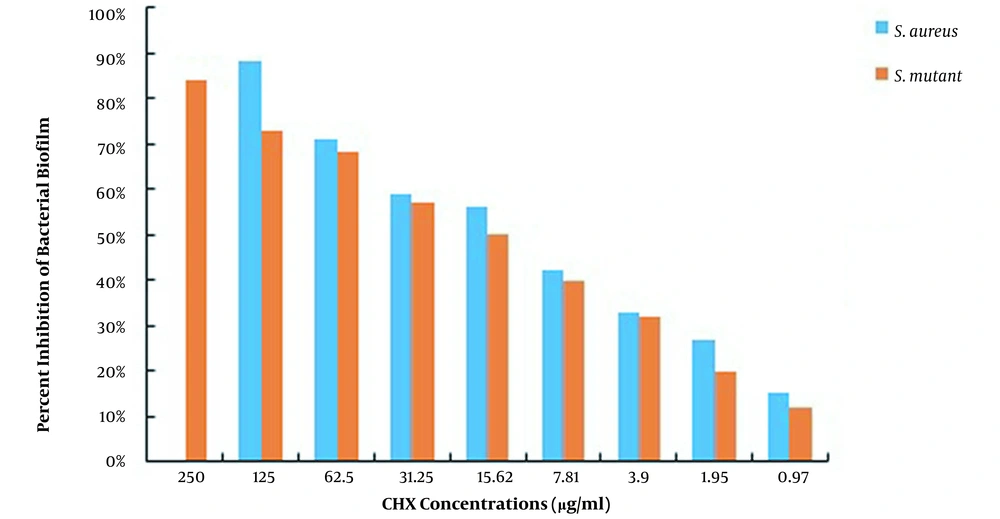
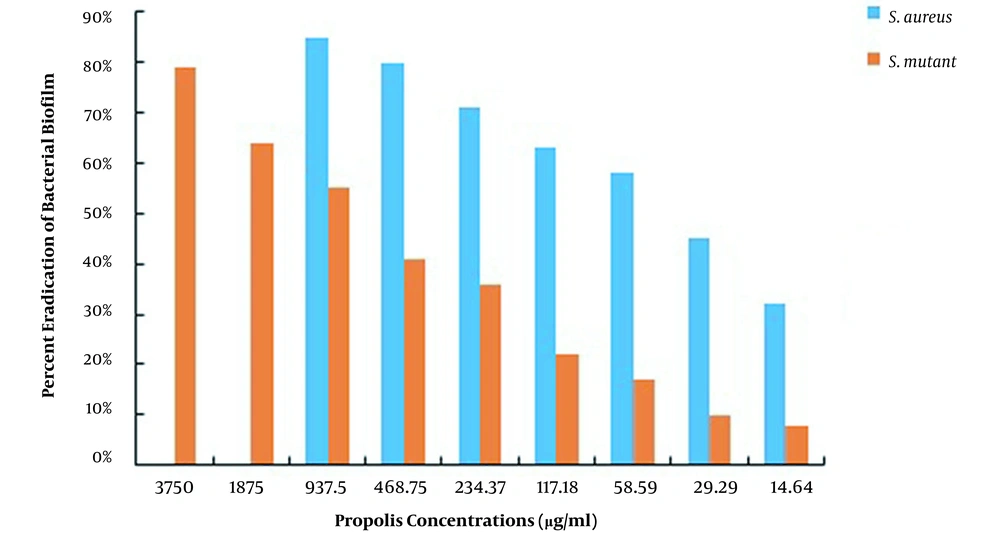
This study used S. aureus and S. mutans to determine the MBEC of CHX and propolis, as shown in Table 2. The MBEC of CHX for S. aureus and S. mutans was 250 and ≥ 500 μg/mL, while the MBEC of propolis was 1875 and ≥ 7500 μg/mL, respectively. After measuring the absorbance at 490 nm, the percentage of biofilms remaining in each well was calculated using the formulas presented, and the percentages of S. aureus and S. mutans biofilm eradication by CHX and propolis aqueous extract were computed, as shown in Figures 3 and 4, respectively. The lowest concentration of CHX and propolis aqueous extract that reduced absorbance by at least 90% was considered MBEC.
| Antibacterial Agent | Range of Concentration (µg/mL) | MBEC (µg/mL) | |
|---|---|---|---|
| Streptococcus mutans | Staphylococcus aureus | ||
| CHX | 0.97 - 500 | ≥ 500 | 250 |
| Propolis | 14.64 - 7500 | ≥ 7500 | 1875 |
4.3. Results of the Synergistic Effect of Propolis Aqueous Extract and CHX 0.2% on MBIC
The results of the synergy study of CHX and propolis aqueous extract are shown in Table 3. The FBICs of CHX and propolis aqueous extract were calculated to be 15.62 and 117.18 μg/mL for S. mutans and 7.81 and 58.59 μg/mL for S. aureus, respectively. Therefore, it is postulated that the synergistic effects were significant (P < 0.05), and MBICs in combination decreased by 4 folds compared to MBIC alone.
| Bacteria and Treatment | MBIC Alone (µg/mL) | MBIC in Combination (µg/mL) | Synergistic Effect |
|---|---|---|---|
| Staphylococcus mutans | |||
| CHX | 125 | 15.62 | Yes |
| Propolis | 3750 | 117.18 | Yes |
| Staphylococcus aureus | |||
| CHX | 125 | 7.81 | Yes |
| Propolis | 937.5 | 58.59 | Yes |
4.4. Results of the Synergistic Effect of Propolis Aqueous Extract and CHX 0.2% on MBEC
The ƩFMBEC analyses are shown in Table 4. Combining the two studied substances with 0.5 ≤ ƩFMBEC showed a synergistic effect. The results are shown in Table 4. The FMBECs of CHX and propolis aqueous extract were calculated to be 250 and 18.75 μg/mL for S. mutans and 125 and 937.5 μg/mL for S. aureus, respectively. In general, the results showed that the combination of the two substances studied had an indifferent effect.
| Bacteria and Treatment | MBEC Alone (µg/mL) | MBEC in Combination (µg/mL) | Synergistic Effect |
|---|---|---|---|
| Staphylococcus mutans | |||
| CHX | ≥ 500 | 250 | Indifferent |
| Propolis | ≥ 7500 | 18.75 | Indifferent |
| Staphylococcus aureus | |||
| CHX | 250 | 125 | Indifferent |
| Propolis | 1875 | 937.5 | Indifferent |
5. Discussion
Microbial biofilms are a community of microorganisms stacked together by a structural matrix (31, 32). Dental plaque is an example of a microbial biofilm that contains layers of growing microorganisms, epithelial cells, macrophages, and leukocytes held together by an organic matrix (33). Dental plaque is the leading cause of periodontal disease, gingivitis, and caries (34). Among the critical microorganisms forming oral biofilms are S. mutans, S. aureus, Lactobacillus spp., E. faecalis, and Candida species (33). There are several mechanisms for controlling biofilm formation: (1) preventing the formation and growth, (2) destroying the biofilm structure, and (3) killing the bacteria that live in the biofilm structure. Among these mechanisms, the prevention of biofilm formation, growth, and colonization using antibacterial agents is the most effective method (35).
In the present study, the effect of different concentrations of CHX and propolis aqueous extract on the inhibition and eradication of biofilms of two caries bacteria, S. mutans and S. aureus, was investigated using the microdilution broth method. The results showed that the MBICs of CHX and propolis aqueous extract were 125 and 3750 μg/mL for S. mutans and 125 and 937.5 μg/mL for S. aureus, respectively.
The results of CHX treatment alone showed that the percentages of S. mutans and S. aureus biofilm inhibition were > 85% (62.5 μg/mL) and > 91% (62.5 μg/mL), respectively. Also, propolis treatment alone showed that the percentages of S. mutans and S. aureus biofilm inhibition were > 81% (1,875 μg/mL) and > 90% (468.75 μg/mL), respectively. Besides, the percentages of S. mutans and S. aureus biofilm eradication by CHX alone were > 84% (250 μg/mL) and > 88% (125 μg/mL), respectively. Also, the percentages of S. mutans and S. aureus biofilm eradication by propolis treatment alone were > 79% (3750 μg/mL) and > 85% (937.5 μg/mL), respectively. As the results showed, the effects of propolis aqueous extract on the percentage of biofilm inhibition and eradication were not significantly different from the corresponding CHX effects, and propolis aqueous extract could affect the biofilm formation and eradication by the studied bacteria approximately as much as chlorhexidine could.
Numerous studies have been performed to compare the antibacterial and anti-biofilm activity of propolis and CHX with other compounds. In 2016, Akca et al. studied the antimicrobial effect of propolis ethanolic extract and CHX gluconate on planktonic and biofilm cells of S. mutans, Streptococcus sobrinus, Lactobacillus acidophilus, and Lactobacillus salivarius. They reported that both ethanolic extract of propolis (EEP) and CHX inhibited the growth of all planktonic species. On the other hand, CHX showed lower bactericidal concentrations than EEP against the biofilms of A. actinomycetemcomitans, S. aureus, and E. faecalis, while EEP had better results against Lactobacillus and P. intermedia. Their results suggested that EEP could be as effective as CHX on oral microorganisms in their biofilm state (20).
Koo et al. in 2002 reported that propolis significantly reduced dental plaque in volunteers (36) and Hayacibara et al. in 2005 reported that propolis prevented plaque formation and caries (37). In 2000, Koo et al. examined the antimicrobial activity of propolis and arnica in vitro against oral pathogens and reported that propolis showed an inhibitory effect on the growth and adhesion of S. mutans (38). Franca et al., in 2014, developed a new varnish based on propolis and chitosan with antimicrobial activity against oral pathogens similar to or even better than CHX varnish (39).
In 2021, Stahli et al. examined the antimicrobial activity of propolis against cariogenic microbial species, periodontal disease, and Candida infections. They used two ethanolic extracts of Brazilian and European propolis (EEP). They reported that European EEP had slightly higher MICs than Brazilian EEPs, with European EEPs showing the most potent effect on biofilm formation delays, while Brazilian EEPs were more active against prefabricated biofilms. According to propolis's antimicrobial and antibiofilm activities, this substance can be used to supplement oral health care products (21). In 2017, Jaiswal et al. examined the antibacterial effects of chitosan, propolis chlorhexidine, and sodium hypochlorite on the biofilm of E. faecalis and reported that chitosan, chlorhexidine, and propolis were as effective as sodium hypochlorite (40).
In 2022, Baldino et al. investigated the effect of nystatin on the efficacy of CHX against S. mutans in plankton cells and biofilms mixed with C. albicans. They reported that nystatin interfered with the action of CHX against S. mutans. The antimicrobial effect of drug combinations depends on their concentration, the time interval used, and the planktonic or biofilm behavior of the microorganisms (41).
In the present study, in addition to a comparative study of the anti-biofilm effects of propolis aqueous extract and CHX on inhibiting and eradicating biofilm formation of the studied bacteria, and their synergistic effects on the anti-biofilm behavior were investigated. The results showed that propolis aqueous extract and CHX had a synergistic effect (P < 0.05) on inhibiting the formation of bacterial biofilms, but both compounds acted indifferently to each other in eradicating bacterial biofilm. There are few studies in this area. In 2017, Ariamanesh et al. examined the synergistic effect of persica mouthwash and ethanolic propolis extract against biofilms of oral pathogens. Their study used a combination of persica, propolis, and honey alone to inhibit the biofilm. The group reported that the combination of persica and propolis was better than honey (33).
5.1. Conclusions
Based on our results, it can be concluded that the use of propolis aqueous extract in appropriate concentrations is effective in biofilm inhibition and eradication of biofilm-producing bacteria such as S. mutans and S. aureus. Although CHX is one of the most common oral disinfectant products against a wide range of microorganisms, propolis may also be used as a mouthwash with reliable natural antimicrobial and biofilm properties to replace or use in combination with CHX as a moderator of its side effects. Of course, in vivo studies are needed to find the effective mechanisms of propolis and its appropriate dose on biofilms.