1. Background
Vitamin D3 deficiency is widespread worldwide and is increasingly growing in the elderly (1). Vitamin D3 is a lipophilic hormone with an essential role in calcium and phosphorus homeostasis (2-5). Serum vitamin D3 level of 30 - 80 ng/mL is considered as normal, 10 - 30 ng/mL as insufficient, < 10 ng/mL as deficient, and > 80 ng/mL as toxic (1).
In healthy adults, high vitamin D3 is linked with reduced prevalence of metabolic syndrome (MetS), dyslipidemia, abdominal obesity, and hyperglycemia (6-8). Therefore, people with lower vitamin D3 levels due to vitamin D3 deficiency show high blood sugar and insulin resistance. They are also at risk of developing metabolic disorders (1). Many studies found an association between vitamin D3 levels and the risk of cardiovascular diseases (9, 10), diabetes (11, 12), hypertension (13), dyslipidemia (1), obesity, and glucose intolerance. In addition, several studies suggested a correlation between vitamin D3 deficiency and MetS (6, 14, 15). Moreover, vitamin D3 deficiency appears to reduce the levels of intracellular calcium and insulin secretion, which results in abnormal beta-cell function and glucose tolerance impairment (16).
Iran is a country with high prevalence of vitamin D3 deficiency, so that it is estimated that nearly 70% of the Iranian population is deficient in vitamin D3 (17). Today, vitamin D3 deficiency has been recognized as a factor in changes in the metabolic function of many cells, including pancreatic cells (18, 19), and people suffering from MetS have the lowest levels of vitamin D3 (20). Clinical and empirical evidence suggests that serum vitamin D3 levels may be inversely associated with several cancers, type II diabetes, MetS, and cardiovascular diseases (19, 21).
While Strange et al. found a link between vitamin D3 deficiency and MetS and its components (risk factors), Bonakdaran et al. showed the lack of a significant correlation between vitamin D3 deficiency and MetS and its risk factors (21, 22).
It has also been claimed that vitamin D3 deficiency is related to increased systolic and diastolic blood pressures (SBP and DBP), as well as higher blood glucose levels. Additionally, vitamin D3 deficiency in women has been shown to be associated with body mass index (BMI) and central obesity, while the correlation between vitamin D3 deficiency and MetS components has not been found to be significant in men (23). Due to the contradictory results regarding the relationship between vitamin D3 and MetS and its components, the existence of the such relationship is still unclear.
In recent years, the analytical performance of immunoassay methods showed highly unstable results. So, liquid chromatography-tandem mass spectrometry (LC-MS/MS) continues to serve as the gold standard to quantitatively determine the vitamin D metabolites in circulation. The results of previous studies showed a good correlation between the high-performance liquid chromatography (HPLC)-UV and the LC-MS/MS methods. The price of an LC-MS/MS device is high; it is also complicated to use and requires expert technicians. These limitations have restricted its use as a routine laboratory method (24).
2. Objectives
So far, few studies have examined the relationship between the level of vitamin D3 and MetS in Mashhad, Iran. To fill this gap, using the HPLC-UV method, we conducted this study to evaluate the relationship between serum vitamin D levels and MetS in patients referred to the central laboratory of Academic Center for Education, Culture, and Research (ACECR) in Mashhad, Iran in 2018.
3. Methods
3.1. Study Population and Design
This cross-sectional study included 1,214 patients referred to the central laboratory of ACECR in Mashhad, Iran, in 2018. The inclusion criteria were age range of 15 - 75 years and having a history of MetS according to the Adult Treatment Panel III (ATPIII) criteria (25). The exclusion criteria included suffering from systemic diseases, pregnancy, lactation, taking anti-dyslipidemia, anti-hypertensive, anti-diabetic drugs, and nutritional supplements during the study. Accordingly, 4 patients were excluded.
All participants were asked to complete a questionnaire that collected information on sociodemographic status, medical history, employment status, smoking habit, amount of daily exercise, and nutrition. The questionnaire was then reviewed by experienced and trained interviewers.
Body mass index, waist circumference (WC), height, and weight of all the subjects were measured using standard protocols. Systolic and diastolic blood pressures were measured using a standard sphygmomanometer on the left arm following a 15-min rest in a sedentary position.
This study was conducted on subjects diagnosed with MetS based on the ATPIII guideline, which is defined according to abdominal obesity (an abdominal circumference of over 102 cm for men and over 88 cm for women) as a mandatory component for the diagnosis of MetS. Moreover, the ATPIII requires at least 2 of the following four features: High triglyceride (150 mg/dL or more), low HDL-c (less than 40 mg/dL in men and less than 50 mg/dL in women), high blood pressure (85/130 mmHg or higher), and high blood sugar (100 mg/dL or higher).
After measuring 25-hydroxyvitamin D (25 (OH) D3), the subjects were divided into 4 groups according to their level: Normal (30 - 80 ng/mL), insufficient (10 - 30 ng/mL), deficient (< 10 ng/mL), and toxic (> 80 ng/mL).
3.2. Blood Sampling
Blood samples were collected into plain plastic tubes in the morning after 12 - 14 hours of fasting, and the hemolyzed samples were excluded from the analysis. Separation of serum from blood samples was done by centrifuging at 4000 g for 15 minutes.
3.3. Measurements of Biochemical Parameters
Serum fasting blood sugar (FBS) and lipid profile were assessed using the Clinical Chemistry Analyzer (Mindray BS800M, China), and 25 (OH) D3 levels were measured using the HPLC method (RIGOL L-3000, China and Agilent, USA). Chromatography was performed with a mobile phase of methanol: water (95: 5 V/V) in Rigol and acetonitrile: methanol (87: 13 V/V) in Agilent and a column temperature of 40°C.
3.4. Statistical Analysis
The statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software version 24. The Kolmogorov-Smirnov test was used to assess the normal distribution of the variables. The quantitative data was presented as mean ± standard division (SD) (for variables with normal distribution) or median and interquartile range (for variables without normal distribution). A P value < 0.05 was considered statistically significant. The chi-square and Fisher’s exact tests were also employed to compare the relationship between vitamin D3 and MetS, as well as the associated risk factors in the groups.
4. Results
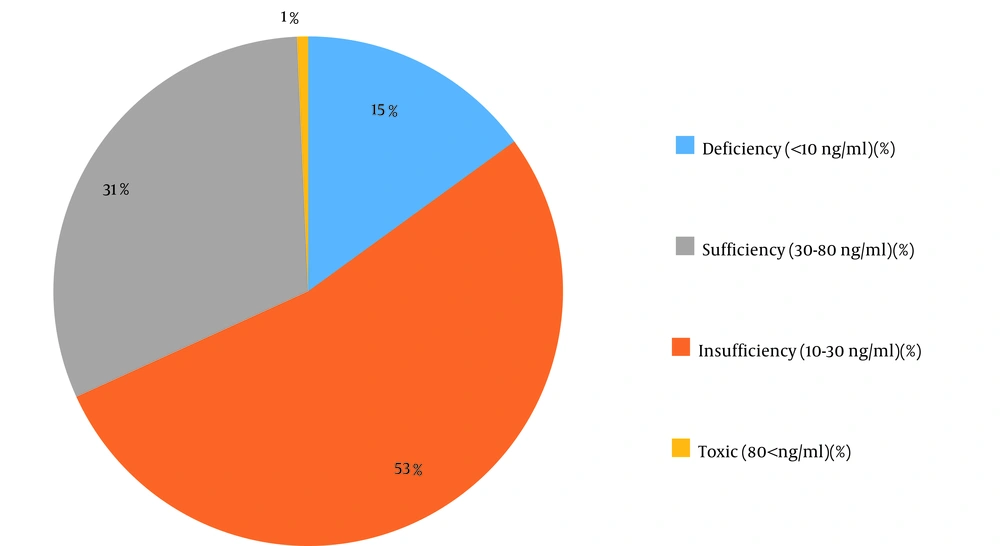
During the study period, a total of 1,214 subjects were referred to the central laboratory of ACECR in Mashhad, Iran. The patients’ demographics are shown in Table 1. The subjects were classified into the following groups based on serum vitamin D3 levels: Deficient (< 10 ng/mL), insufficient (10 - 30 ng/mL), sufficient (30 - 80 ng/mL), and toxic (> 80 ng/mL). As shown in Figure 1, 15.0% of the subjects had deficiency, 53.2% had insufficiency, 31.1% were normal with sufficient vitamin D3 levels, and 0.7% suffered from toxicity (a high serum level of vitamin D3). In addition to classifying the subjects based on their vitamin D3 serum levels, the patients were divided into groups according to the MetS status. Furthermore, the relationship between these conditions and gender was separately assessed (Tables 2 and 3). Among the study population, 243 females and 111 males had MetS aside from their vitamin D3 status.
| Variables (n = 1214) | Minimum | Maximum | Mean ± SD |
|---|---|---|---|
| Age | 15 | 75 | 41.17 ± 14.04 |
| SBP (mmHg) | 8.0 | 20.0 | 11.84 ± 1.63 |
| DBP (mmHg) | 4.0 | 12.0 | 7.41 ± 1.09 |
| WC (cm) | 44 | 147 | 89.45 ± 11.97 |
| BMI (kg/m2) | 15.24 | 57.44 | 27.56 ± 5.24 |
| Height (cm) | 134.0 | 198.0 | 162.55 ± 9.79 |
| Weight (kg) | 40.80 | 143.0 | 72.72 ± 14.69 |
Demographic Results of Patients
| Variables | Vitamin D3 (25 (OH) D) | |||
|---|---|---|---|---|
| Deficiency (< 10 ng/mL) | Insufficiency (10 - 30 ng/mL) | Sufficiency (30 - 80 ng/mL) | Toxic (> 80 ng/mL) | |
| Sex | ||||
| Female | 153 (17.4) | 437 (49.7) | 283 (32.2) | 7 (0.8) |
| Male | 29 (8.7) | 209 (62.6) | 94 (28.1) | 2 (0.6) |
| Total | 182 (15.0) | 646 (53.2) | 377 (31.1) | 9 (0.7) |
As shown in Table 4, subjects in the two groups of MetS and non-MetS did not have a significant difference in serum levels of vitamin D3 (P = 0.608). Furthermore, population-based data was used to characterize the serum metabolite profile associated with vitamin D3 levels in the total population (Table 5). Next, the situation was evaluated in the group with MetS. The relationship between serum vitamin D3 levels and the risk factors of MetS with serum metabolites was also assessed. According to Table 5, there was a significant direct correlation between vitamin D3 levels and SBP in the group with MetS, but there was no significant association between serum vitamin D3 and other risk factors of MetS.
| Variables | Vitamin D3 (25 (OH) D) | |||
|---|---|---|---|---|
| Deficiency (< 10 ng/mL) | Insufficiency (10 - 30 ng/mL) | Sufficiency (30 - 80 ng/mL) | Toxic (> 80 ng/mL) | |
| Non-metabolic syndrome | 135 (15.7) | 458 (53.3) | 260 (30.2) | 7 (0.8) |
| Metabolic syndrome | 47 (13.3) | 188 (53.1) | 117 (33.1) | 2 (0.6) |
| Total | 182 (15.0) | 646 (53.2) | 377 (31.1) | 9 (0.7) |
| Variables | Total Correlation | ||||
|---|---|---|---|---|---|
| Serum Vitamin D3 (25 (OH) D) in Total Population | Serum Vitamin D3 (25 (OH) D) in Subjects with Metabolic Syndrome | ||||
| Deficiency (< 10 ng/mL) | Insufficiency (10 - 30 ng/mL) | Sufficiency (30 - 80 ng/mL) | Toxic (> 80 ng/mL) | ||
| FBS | -0.096 | 0.020 | -0.042 | 0.242 | 0.033 |
| TG | 0.021 | -0.012 | -0.059 | -0.044 | -0.48 |
| Chol | 0.076 | 0.009 | 0.021 | 0.062 | 0.093 |
| HDL | 0.006 | -0.048 | 0.073 | 0.342 | 0.037 |
| LDL | 0.044 | 0.003 | 0.020 | 0.028 | 0.057 |
| Ca | -0.026 | -0.011 | 0.037 | 0.089 | 0.014 |
| P | 0. 122 | -0.021 | 0.027 | 0.079 | -0.057 |
| SBP | 0.049 | 0.023 | 0.030 | 0.637 | 0.131 a |
| DBP | -0.010 | -0.018 | -0.018 | -0.597 | 0.57 |
| WC | -0.51 | -0.011 | -0.050 | -0.286 | -0.051 |
| BMI | -0.032 | 0.035 | -0.024 | -0.461 | 0.059 |
Correlation Between Metabolic Syndrome Risk Factors and Serum Vitamin D3 Levels in 4 Groups of Serum Vitamin D3 and Subjects with Metabolic Syndrome
5. Discussion
This study aimed to determine the prevalence of vitamin D3 deficiency and MetS and to evaluate the association between them. A high proportion of our subjects had abnormally low vitamin D3 levels. Also, 68.2% of the population suffered from insufficient vitamin D3 levels (< 30 ng/mL). In agreement with this study, several previous studies revealed the high prevalence of vitamin D3 deficiency in the Iranian population (26-30), which is due to various factors like the way of dressing, having unhealthy dietary habits, and overuse of cosmetics and sunscreens (31-33). The high degree of discrepancy in various studies may be ascribed to the following factors: Different study populations, ethnic diversity in vitamin D3 metabolism, genetic and epigenetic factors, the season in which the studies were performed, as well as the methods used for determining vitamin D3 levels (34-37). In our subjects, the prevalence of vitamin D3 insufficiency in males was higher than that of females, partly due to the fact that a growing number of women are taking vitamin D3 supplements, which is consistent with the results of previous studies (38). In contrast, some other studies showed that women are more vitamin D3 deficient than men (39, 40).
In our study, the prevalence of MetS was 29.2%. The results of a study by Kalan Farmanfarma et al. showed that approximately one-third of the adult Iranian population has MetS (41). It has also been suggested that MetS can be influenced by different factors such as female gender, aging, and being overweight or obese (42). In this study, there was no significant difference in serum vitamin D3 levels between participants with and without MetS. Similar to this study, Bonakdaran et al. found no considerable difference in serum vitamin D3 concentrations between individuals with or without MetS (22). Kaseb et al. found no significant association between MetS and vitamin D3 deficiency among overweight and obese adults (43). Liu et al. stated that neither total nor supplemental vitamin D3 was significantly related to MetS (5). Reis et al. studied participants aged 44 - 96 years and found no correlation between vitamin D3 and MetS in either sex (44). Several studies support the hypothesis that adequate blood levels of vitamin D3 improve MetS (45, 46). Rafraf et al. showed that subjects with MetS had significantly lower vitamin D3 levels than those without MetS (47). Esteghamati et al. found that metabolically healthy obese subjects had higher serum vitamin D3 levels than those with metabolically unhealthy obesity (48). Ford et al. showed that the concentration of vitamin D3 was significantly lower among patients with MetS than those without MetS (15). Chiu et al. studied healthy glucose-tolerant subjects and showed that those with hypovitaminosis D3 had a higher prevalence of MetS components than subjects without hypovitaminosis D3 (49). Botella-Carretero et al. reviewed morbidly-obese patients and showed that vitamin D3 deficiency was more prevalent in morbidly-obese patients that suffered from MetS compared with those who did not meet the criteria for this syndrome (50). Lu et al. found that low vitamin D3 level was significantly associated with an increased risk of MetS (51).
The relationship between serum vitamin D3 and MetS risk factors is shown in Table 5. According to our result, there was a significant direct correlation between serum Vitamin D3 levels with SBP in subject with MetS, but no significant associations were found between serum vitamin D3 and other risk factors of MetS. Contrary to this result, Rafraf et al. showed that serum vitamin D3 was inversely associated with FBG, and no significant associations were found between serum vitamin D3 and other risk factors of MetS (47). Furthermore, Chiu et al. found a negative correlation between vitamin D3 concentration and BMI, total and LDL cholesterols, and 60-, 90-, and 120-min post-challenge plasma glucose concentrations (49). Among the components of MetS, a significant inverse correlation was found for quintiles of vitamin D3 concentration with abdominal adiposity, hypertriglyceridemia, and hyperglycemia (15). In another study, serum vitamin D3 level was negatively and significantly associated with WC, SBP, and DBP, but positively associated with BMI (26).
This study had several limitations. First, the cross-sectional nature of the data impedes the ability to infer the causation between vitamin D3 and the risk factors of MetS. Second, a relatively small sample size was another limitation of this study. Therefore, researchers can conduct more studies with larger sample sizes.
5.1. Conclusions
According to the results of this study, a high ratio of our subjects suffered from MetS (29.2%) and insufficient vitamin D3 levels (68.2%). Also, there was no significant difference between serum vitamin D3 levels in participants with MetS and those without MetS. Future studies with a long-term longitudinal design are needed to explain the cause-and-effect relationship between vitamin D3 status and MetS.