1. Background
Colon cancer is a major health concern in the modern world. It is the second cause of cancer-related mortality in western countries and the fourth cause of cancer-related mortality worldwide. It is estimated that there are 1.2 million new colon cancer cases each year, which accounts for 9.7% of all cancer cases worldwide. Colon cancer is the fourth most common cancer in Iran, ranking fourth among men and second among women (1, 2). Aside from inherited genetic factors, sedentary lifestyle, consumption of red meat and processed meat, substantial alcohol intake, and body fat, especially abdominal fat, are thought to be some of the most important risk factors for this disease. Some common symptoms of colon cancer include continuous abdominal pain, nausea, vomiting, bleeding, colon rupture, and colon obstruction. Colonoscopy, computed tomographic colonography, and biopsy are the most recommended diagnostic methods for colon cancer worldwide. Age, gender, presence of symptomatic tumors, tumor grading, prior clinical history, and chemotherapy experience, and individual response to treatment are among the most important prognostic factors (3). Chemotherapy is one of the recommended treatments for colon cancer patients, especially patients with III-stage colon cancer or II-stage patients that are at high risk. Oxaliplatin, 5-fluorouracil, capecitabine, and leucovorin are some of the chemotherapy drugs used to treat these patients. However, these drugs often cause detrimental side effects, such as vomiting, diarrhea, weakened immune system, hair loss, and weight loss. Therefore, scientists have been searching for alternative drugs with fewer side effects to improve the recovery of colon cancer patients after therapy as well as their overall health both during and after the treatment. To improve the patient’s quality of life, traditional medicine, or natural medicine as it is called in some literature, has been the main focus (4-6).
Traditional medicine has been an important part of human evolution and has been a crucial part of our culture. Different traditional remedies have intermingled and blossomed into a sophisticated branch of medicine. It has been the main means of treatment for various diseases, including cancer, throughout human history (7, 8). Herbal remedies have been an essential part of traditional medicine to fight against diseases, especially cancer (9). Ocimum basillicum (OB) or basil, is a member of the mint family which is native to tropical zones. Its components are reliant on where it has been cultivated. This crop is rich in linalool, eugenol, 1,8-cineone, methyl eugenol, and anthocyanins, which are mainly responsible for its pharmaceutical effectiveness. OB has been used as an effective treatment for a variety of health complications throughout history. Basil has been used to treat asthma, colds, anorexia, arthritis, etc. It is effective against viral, bacterial, and fungal infections as well. OB has anti-cancer, anti-inflammatory, anti-pyretic, antioxidant, cytotoxic, and immunomodulatory effects (10, 11). Considering the medicinal properties of the OB mentioned above and the immediate need for alternative and less harmful treatment methods for colon cancer, in this study, we analyzed the anti-proliferative effect of OB leaf aqueous extract on human colon cancer cell lines LS174T and COLO205 and the expression of anti- and pro-apoptotic genes compared to normal colon cell line HCT116.
2. Objectives
The goal of this study was to investigate the effect of OB leaf aqueous extract on mentioned cell lines in comparison with untreated cells of the same type to deduce if this extract can inhibit the proliferation of these cancer cells and induce apoptosis by increasing the expression of pro-apoptotic genes, like BAD and BAX.
3. Methods
3.1. Plant Aquos Extract Preparation
OB leaves were gathered from the fields across Tabriz, Iran. Then, the leaves were dried in the dark, air-dried for a week before being completely ground. Then, 300 gr of the resulting powder was mixed in 1800 mL (1 to 6 weight/volume ratio) distilled water while being heated at 40°C simultaneously for 2 hours. Then, the solution was left at room temperature for 24 hours before being filtered with Whatman paper number 2. The primary extract was evaporated at 80°C for 1 hour in a vacuum distillation unit (rotary with a vacuum pump), and the concentrated extract was collected. The extract was sonicated to form a better and homogenous solution and was filtered through 0.2 µm filters to filter out bacterial pollution. GC-mass was performed to analyze the components within the OB leave extract.
3.2. Cell Culture
Human colon cancer cell lines HCT116, LS174t, and COLO205 were obtained from the Pasteur Institute of Iran. These cells were cultivated in Roswell park memorial institute (RPMI) 1640 medium. COLO205 cells were cultured in gelatine-coated plates for better adherence. Both mediums were supplemented and enriched with 10% fetal bovine serum (FBS), 1% antibiotic mixture (penicillin/streptomycin), 1% L-glutamine, and 1% non-essential amino acids. Cells were incubated in an atmosphere containing 5% CO2 at 37°C.
3.3. Cell Viability
The methyl thiazol tetrazolium (MTT) assay was performed to assess cell viability. The MTT kit under the name cytotoxicity test kit (Arsamsysbio.com, Iran) was used in this assay, and the instructions were followed as mentioned in the kit. Then, 3000 cells were transferred to each well of a 96-well plate with appropriate mediums, and they were incubated for 24 hours. The next day, after changing the medium, cells were treated with different concentrations (2, 4, 6, and 8 mg/mL) of OB extract. These concentrations were selected based on information from similar studies. After 24 and 48 hours of incubation, 10% MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was added to the wells, and the plate was incubated for 4 hours. After incubation, the wells were emptied, and formazan solubilizing solution was added, and absorbance was measured at 560 nm.
3.4. mRNA Extraction
RNA extraction was conducted using the thiazol kit under the brand name ZiAzole (ZiAViZ company, Iran). The cells were added with 1 mL of thiazole. The cells were then placed on ice for 1 minute before being vortexed. The mixture was set at room temperature for 5 minutes. Then, 200 µL of chloroform was added and mixed in for 15 seconds before the mixture was put on ice for 5 minutes, and after that, it was centrifuged at 12000 rpm for 10 minutes. Then, the top aqueous layer was separated from the organic phase, and the phase containing proteins and debris was transported to a new microtube. Afterward, 1 mL of isopropanol was added and slowly shaken before putting on ice for 15 minutes and being centrifuged for 15 minutes at 12000 rpm. The top layer of the resulting mixture was discarded, and 1 mL of 75% ethanol was added to the rest of the mixture. The mixture was shaken slowly and centrifuged again at 12000 rpm for 10 minutes. The top layer was discarded, and the sediment was set down at room temperature until the ethanol is completely evaporated. Then, for resuspension of the sediment, 50 to 100 µL of sterile water was added. The mixture was set down at room temperature for 30 minutes before being stored in the freezer.
3.5. Reverse Transcription (RT) PCR
RT PCR was conducted using the cDNA Synthesis Kit (Yekta Tajhiz, Iran) to obtain cDNA from mRNAs to be used for gene expression assay (qPCR). Five microliters of mRNA were mixed with 1 µL of oligo(dT) primer and 5 µL of sterile water. The amount of RNA used in the test was measured by the NanoDrop spectrophotometer. It was heated for 5 minutes at 70°C and was immediately put on an ice-cold rack. The PCR master mix was prepared according to kit instructions, and 6.5 µL was added to each sample, and the final phase of RT-PCR was performed under the following conditions: one hour of 42°C treatment followed by 5 minutes at 70°C.
3.6. Real-Time PCR Assay
The qPCR assay was conducted to evaluate the expression of BAX and BAD genes. The expression of the βActin gene was considered our reference gene. The qPCR kit (Yekta Tajhiz) was used for this assay, and all the steps were conducted according to the instructions in the kit. The cells were treated with 0.5, 1, 2, and 4 mg/mL of our extract for 48 hours before the assay, and these concentrations were decided considering the results obtained from the MTT assay along with the information from other similar studies. The sequence of the primers is shown in Table 1.
| Gene | Reverse | Forward |
|---|---|---|
| βActin | 5'-CTAGAAGCATTTGCGGTGGA-3' | 5'-GGAGTCCTGTGGCATCCACG-3' |
| BAX | 5'- GCCACGTGGGCGGTCCCAAAGT -3' | 5'- GGCCCACCAGCTCTGAGCAGA-3' |
| BAD | 5'-TCCAGCTAGGATGATAGGAC-3' | 5'-CAGTGATCTGCTCCACATTC-3' |
The qPCR cycle settings were as follows: (1) an initial denaturation period at 95°C for 2 minutes; (2) each cycle was comprised of 15 seconds of denaturation at 95°C, 15 seconds of annealing at 59°C, and 20 seconds of synthesizing at 7°C.
3.7. Data Analysis
All assays were performed three times to ensure the validity of the data. Data analysis and figure preparation were performed using GraphPad Prism 9 (GraphPad Software, CA, USA), and the statistical significance was set at P < 0.05. All results are expressed as mean ± SD.
4. Results
4.1. MTT Assay Results
Analysis was conducted on data gathered from MTT assay in 24 and 48 hours marks for LS174t and COLO205 cells, compared to HCT116 normal cells, treated with different concentrations of OB leaf aqueous extract.
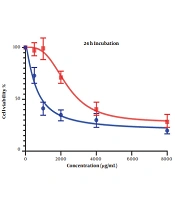
The cell viability changes at the 24-hour mark can be seen in Figure 1. The IC50 values for LS174T and COLO205 cell lines were calculated to be 596.1 and 2331 µg/mL, respectively.
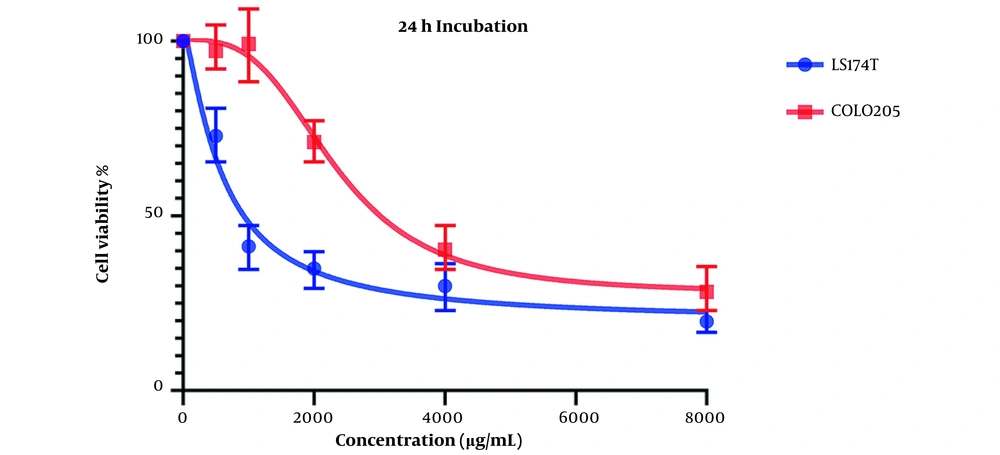
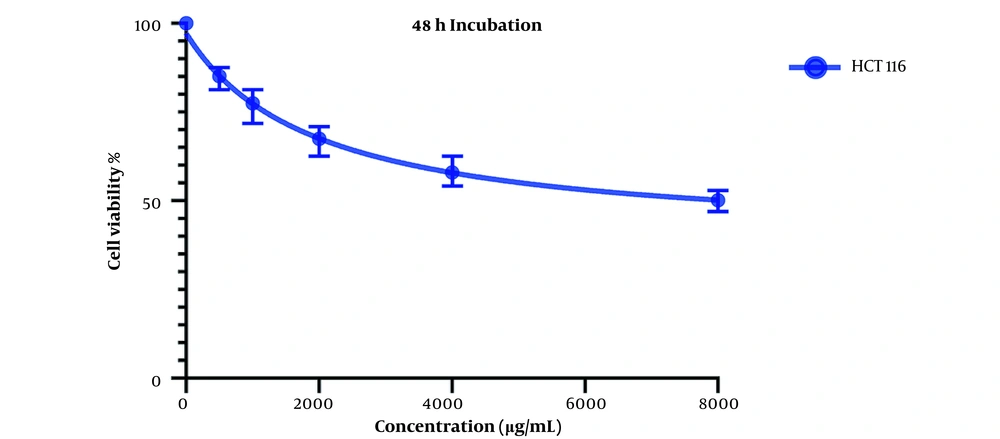
Figure 2 presents the cell viability changes after 48 hours of treatment with various concentrations of OB extract, and the IC50 values for LS174T and COLO205 cell lines were 527.9 and 5862 µg/mL, respectively.
Both results indicate acceptable cytotoxic activity from OB extract in both cell lines, especially on LS174T cell lines. Results also showed that this cytotoxic effect is stronger in higher concentrations of our extract.
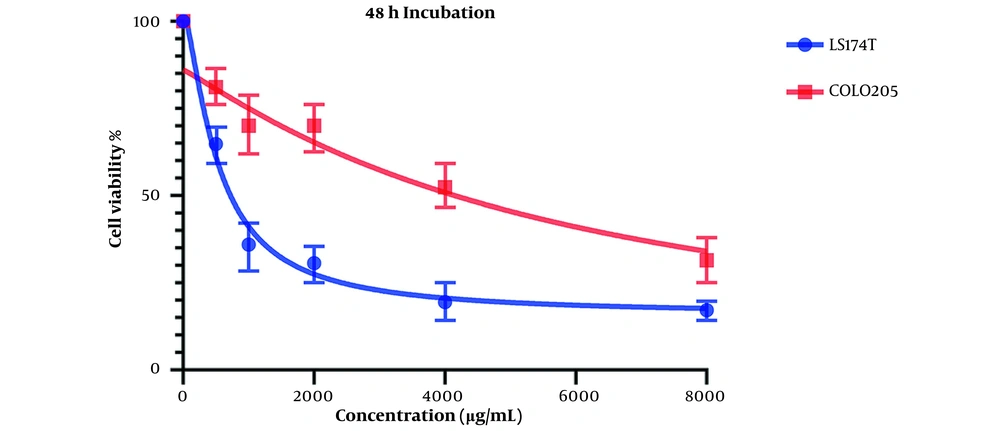
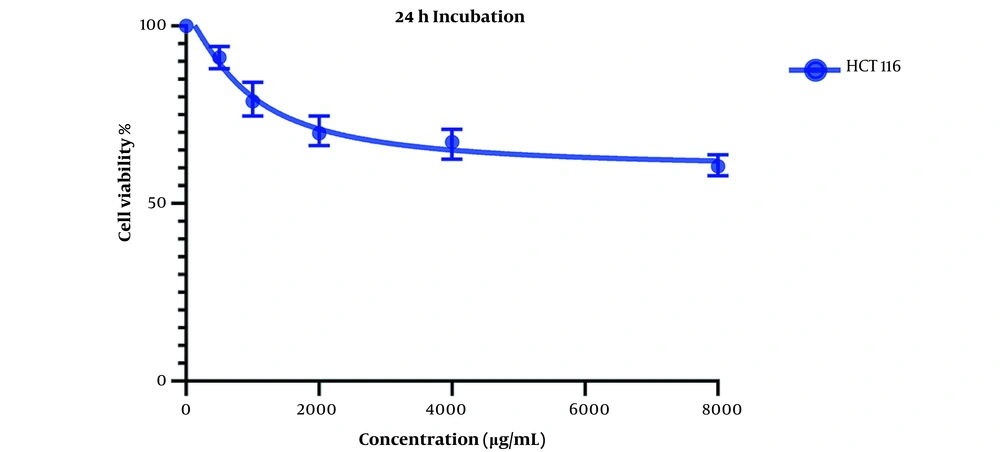
Comparing these results to the results obtained from the MTT assay conducted on HCT116 normal colon cells, in which the IC50 values for the 24-hour mark (Figure 3) and 48-hour mark (Figure 4) were 22164 and 3512 µg/mL, respectively, we can conclude that our extract was severely potent against cancerous cells compared to normal cells. In general, considering the data presented in the graphs, there seems to be a noticeable difference in the viability between the cells that were treated with our extract and the untreated cells, either cancer cells or normal cells, as well as a direct and inverse correlation between the concentration of the extract introduced to the cells and the number of live cells after incubation. It can be concluded that our extract can negatively affect the viability of these cancer cells.
4.2. Real-Time PCR Results
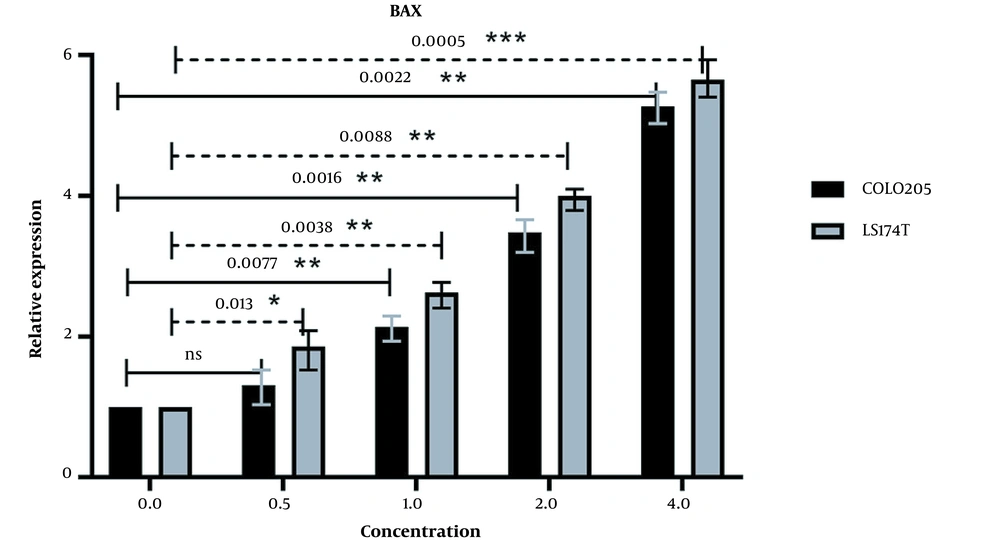
qPCR was conducted to evaluate the expression of BAX and BAD. The expression of these pro-apoptotic genes can be a great indicator of cellular viability. Increased expression of these genes can positively affect apoptosis and lead to the death of cancer cells. The βActin gene was chosen to be our reference gene. The expression of pro-apoptotic BAX was increased in both cell lines (Figure 5), and there was a positive correlation between the increase in extract concentration and BAX expression. The increase of BAX expression in the LS174T cell line was higher, which may be due to the difference in key mutations of these cell lines. LS174T has a K-RAS mutation as opposed to the B-RAF mutation of COLO205 cells, which may cause LS174T cells to be more sensitive to our extract. Calculated p-values (at P < 0.05) show a significant change in the relative expression of the BAX gene in all concentrations in both cell lines except for the 0.5 mg/mL concentration of COLO205 cells.
The relative expression of BAX in LS174T and COLO205 cells after treatment: The expression of BAX increased after treatment with different concentrations of Ocimum basillicum (OB) extract, and the increase in the concentration of OB extract was positively correlated with the increase of BAX expression.
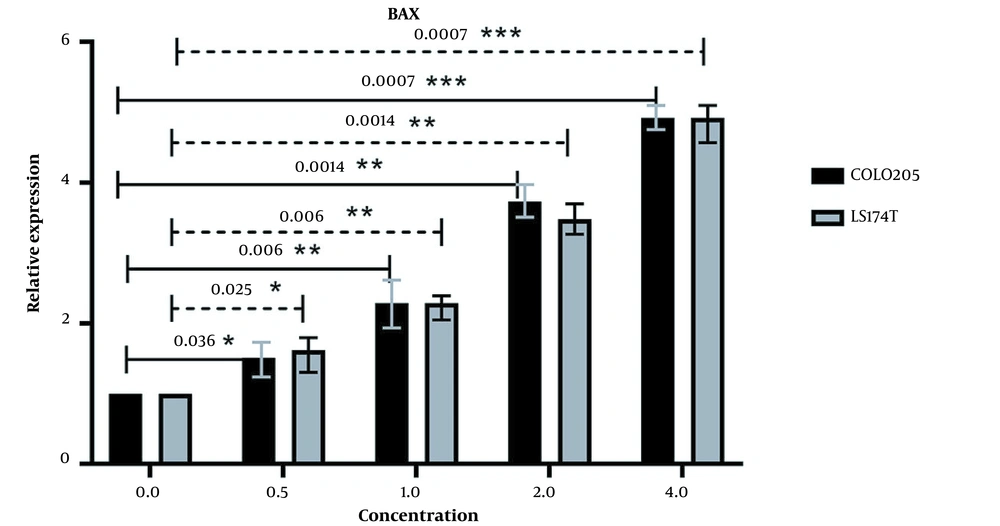
The expression of BAD was also increased after the same treatment process was applied to it (Figure 6), and the increase in expression of BAD also had a positive correlation with the increase in extract concentration. There seems to be a fluctuation in the difference in BAD expression in both cell lines, as seen in Figure 6. But overall, the expression of BAD was increased after treatment with our extract in comparison with untreated cells. P-value (at P < 0.05) analysis confirms the significance of the changes in the expression of this gene in both cell lines.
The relative expression of BAD in LS174T and COLO205 cells after treatment: The expression of BAD increased after treatment with different concentrations of Ocimum basillicum (OB) extract, and the increase in the concentration of OB extract was positively correlated with the increase of BAD expression.
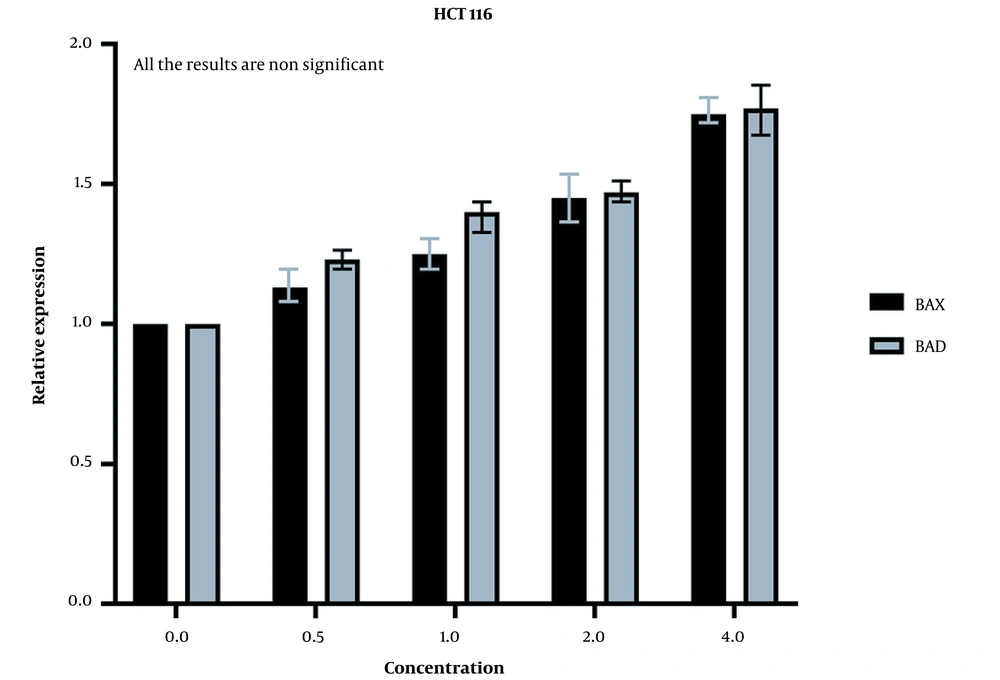
The expression of these genes was increased in HCT116 normal cells as well (Figure 7), but the increase was insignificant according to the statistical analysis. These results mean that our extract cannot induce a strong apoptosis response in normal cells as it does in cancer cells.
The positive correlation between the increased expression of these pro-apoptotic genes and the increasing concentration of our extract as well as the differences in the expression of these genes in treated and untreated cells in cancer cells lines and normal cell lines, demonstrates that the increased expression of BAD and BAX is associated with our extract.
5. Discussion
Colon cancer is a major health risk in modern societies and claims several lives each year. Its growth rate is increasing each year due to lifestyle changes in the modern world. Considering that there are several types of mutations involved in this cancer and that chemotherapy is one of the main treatments for this disease, along with the harmful side effects of chemotherapy, the development of new treatment methods that are safer and have fewer side effects are required. It should be noted that each drug is more effective against a few selected mutations, and this fact should be considered when prescribing these drugs to maximize the effects of therapy. Our study aimed to study the effects of OB leaf aqueous extract on colon cancer cell lines and the expression of apoptotic genes.
In the MTT assay, our extract performed as expected and, according to the results, had good cytotoxicity effects on our cell lines at 24 and 48-hour marks. The cytotoxicity effect was stronger in higher concentrations of OB extract. The cytotoxicity effect was so drastically high in treated cells in comparison with untreated cells, which showed an increase in correlation with increasing concentrations, demonstrating the cytotoxic properties of our extract. One study investigated the effects of OB extract on the human cervical cancer cell line (HeLa), human laryngeal epithelial carcinoma cell line (HEp-2), and NIH 3T3 mouse embryonic fibroblasts through the MTT assay and reported that this extract has strong cytotoxicity effects on all cell lines (12). Results of this study and another study that also reported that this extract has potent cytotoxicity on breast cancer cell lines, MCF-7, and MDA-MB-231 are in line with our findings (13). Hanachi et al. also performed an MTT assay to investigate the effects of some extracts, including OB extract, on AGS gastric adenocarcinoma cell lines and once more proved that our findings are consistent and this extract has cytotoxicity effects on various cancer cell lines (14). In 2018, Torres et al. also studied the effects of OB aqueous leaf extract on MCF-7 breast cancer cell line through an MTT assay and their results reported up to 80% cytotoxic effect on MCF-7 cells. Their study also reported some effects on the proliferation and metabolism of these cells after OB extract treatment (15). Another study that investigated the cytotoxicity of several herbal extracts, including OB extract, was performed by Elgndi et al. This study explored the effects of these extracts on HeLa, MDA-MB-453, and myelogenous leukemia (K562) cell lines and confirmed that OB extract has a cytotoxic effect on all mentioned cell lines. This was confirmed by the MTT assay (16). Taking into account the results of our study and the results of these previous studies, we can safely state that this herbal extract has cytotoxic effects on various cancer cells and can be a viable focus point for further studies.
Our study also showed that the overall effectiveness of OB extract was stronger on the LS174T cell line, which might be due to the different key mutations in these cell lines, as stated before. The MTT results for HCT116 normal colon cells also proved that our extract was not highly harmful to normal cells, which can prove to be a positive point in future clinical settings. However, there were no prior studies on the effects of OB leaf aqueous extract on normal and cancerous cell lines.
Analysis of qPCR data indicated that the expression of pro-apoptotic BAX and BAD increased as expected. The increase in BAX and BAD had a positive correlation with the increased concentration of OB extract used to treat the cells. Furthermore, the expression of BAX increased more in the LS174T cell line, but the expression of BAD was relatively similar in both cell lines. The expression of BAX and BAD showed no significant increase in HCT116 normal cells, which indicates that this extract is not likely to induce apoptosis in normal cells. Behbahani investigated the effects of OB extract on MCF7 and MDA-MB-231 cell lines and the expression of BAX, P53, Caspase 3, and BCL2 genes. The results of this study indicated that the expression of the BAX gene increased in both cell lines, while the expression of the BCL2 decreased in the MCF7 cell line (13). No previous study has investigated the expression of BAD after treatment with OB, but because of the similarity of BAD and BAX actions, we can assume that our results can be duplicated on other cell lines as well. Overall, an increase in the expression of pro-apoptotic genes can initiate the process of apoptosis in cancer cell lines and open up new doors for the effective treatment of cancer. Consiedering the results of our study and multiple other studies, it can be concluded that OB extract can increase the expression of BAD and BAX. This result, along with the results of the MTT assay surmise that this extract has anti-cancer properties on multiple cell lines and colon cancer cell lines LS174T and COLO205 and should be studied in-depth in the future.
5.1. Conclusions
Finding new and effective treatment methods for colon cancer treatment with fewer side effects is very important and urgent. Our results demonstrate that OB leaf aqueous extract has potent cytotoxic activity on colon cancer cell lines LS174T and COLO205 and can elevate the expression of pro-apoptotic BAX and BAD in these cells but is most likely not harmful to normal colon cells. Multiple previous studies also confirm our results and indicate that this extract has cytotoxic and pro-apoptotic properties on other cancer cell lines. Future studies are required to determine the chemical compounds of this extract that are responsible for these effects. Other inquiries are also needed to evaluate the importance of different mutations in the effectiveness of the mentioned extract.