1. Background
Chemotherapy is one of the therapeutic interventions for the treatment and survival of patients with cancer. Doxorubicin (DOX) is an effective anticancer drug against a wide variety of cancers. One of the side effects of DOX is the adverse effects of this drug on spermatogenesis, which increases oxidative stress in men (1). Leydig cells are one of the three types of testicular somatic cells that release most testosterone. These cells produce testosterone through luteinizing hormone (LH), which is released from the anterior pituitary gland in response to gonadotropin-releasing hormone from the hypothalamus. Luteinizing hormone binds to its receptor and facilitates the transport of cholesterol to the mitochondria. Eventually, it leads to the synthesis and secretion of testosterone by Leydig cells (2).
It seems that any factor that affects the increase in the number of LH receptors (LHR) on Leydig cells increases the response of Leydig cells to LH, thus affecting testosterone secretion and spermatogenesis, which includes spermatocyte proliferation, spermatogonia to spermatocyte differentiation, spermatid production, and sperm maturation. Studies show that side effects of chemotherapy on Leydig cell dysfunction are associated with increased LH levels and no change or decrease in testosterone levels (1).
Due to the toxicity of DOX on testicular tissue, much research has been conducted on supplementary medicine, especially herbal medicine and other biologically active compounds (3). Crocin (Cr) is the main active ingredient of saffron with a range of medicinal properties such as anti-inflammatory, antioxidant, anti-degenerative, and anticancer properties (4). The results of some in vitro and in vivo studies have shown that Cr consumption has a protective role in the treatment of cancer using DOX (5-7); however, our current knowledge of the effect of this supplement on Leydig cell damage and infertility from DOX is limited (8). Some studies have reported that exercise has a positive role on sperm-related parameters and spermatogenesis-related signaling (9, 10), but some studies have reported the negative effects of exercise on spermatogenesis performance (11).
2. Objectives
Due to the lack of research on the effect of high-intensity interval training (HIIT) and Cr and their side effects of DOX chemotherapy on testicular tissue, this study aimed to investigate the effect of HIIT and Cr on doxorubicin-induced damage to spermatogenesis and LH receptors in the Leydig cells of male Wistar rats.
3. Methods
3.1. Animals
Thirty male Wistar rats (8 weeks) weighing approximately 220 ± 20 gr were purchased from the Laboratory Animal Breeding Research Center of Ahvaz Jundishapur University of Medical Sciences and were kept under standard conditions (temperature 25 ± 2°C, relative humidity 50 ± 5%, 12: 12-hour light-dark cycle and free access to water and food). All procedures were performed on animals in accordance with the guidelines of the National Ethics Committee in Biomedical Research and the Code of Ethics, IR.SSRC.REC.1399.026 of the Sport sciences research Institute.
3.2. Study Groups and Treatment of Animals
Animals were randomly divided into five groups (n = 6), including (1) healthy (control), (2) DOX (model), (3) doxorubicin + 10 mg/kg body weight (kg.bw) Cr (DOX-Cr10), (4) DOX + HIIT (DOX-HIIT), and (5) DOX + HIIT with 10 mg/kg.bw Cr (HIIT-Cr10).
DOX-Cr10 and HIIT-Cr10 groups received daily Cr (Sigma-Aldrich Co) at specific doses. Crocin supplement (St. Louis, MO, USA) with a purity of 98% was diluted in 1-g vial with normal saline, and 10 mg/kg rats body weight was administered orally at 10 am after the training sessions for eight weeks. Also, during the study period, all groups except the control group received a dose of two mg/kg of doxorubicin (Belgian Abve Company) dissolved with normal saline peritoneally seven times on Fridays (48 hours after the last training session). At the same time, the control group received two mg/kg of normal saline through a nasogastric tube (12).
3.3. Exercise Training Procedure
Before starting the main protocol, rats trained (10 days) for five to 10 minutes each time to get acquainted with the training protocol. The maximum training speed (Vmax) was determined after performing an incremental test (13). High intensity interval training protocol was performed five days/week for eight weeks and consisted of three parts: warm-up, cool-down (five minutes at a 50 to 60% Vmax), and main training (14).
3.4. Animals’ Dissection and Sampling
After 12 hours of fasting and 24 hours of the training session and oral administration of Cr supplementation, rats were anesthetized with intraperitoneal injection of ketamine 10% (50 mg/kg.bw) and xylazine 2% (10 mg/kg.bw) after five minutes. Testicular tissue samples were dissected. The rats’ testicular tissues were allowed to be fixed in 10% buffered formalin solution for six hours and then processed.
3.5. Hematoxylin and Eosin Staining
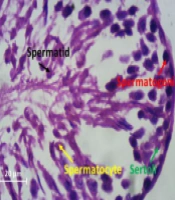
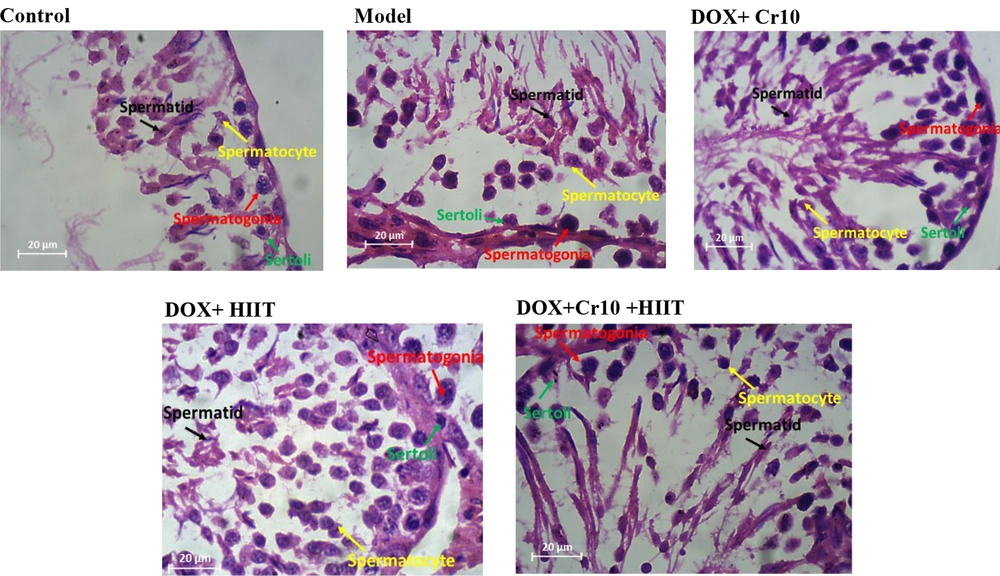
First, 10% buffered formalin solution was used to fix the tissue. Then the steps of dehydrating, clarifying, and soaking in paraffin were performed. Then molding and cutting were done with a microtome machine with a thickness of five micrometers. The slices are placed on albumin material. The slices were first deparaffinized, then clarified and hydrated with ethyl alcohol. After that, the slides were placed in hematoxylin color for 15 minutes, and after removing the slides, they were washed with running water for five minutes. To fix the nuclear color, the slides were placed in lithium carbonate for three minutes. Again, the samples were washed with running water and placed in eosin for 10 minutes. In the next stage of dehydration with ascending degrees of ethyl alcohol for one minute, then the slides were placed in xylol twice for 15 minutes. Nuclei counts were randomly counted with a 40-microscope lens with a magnification of 400 with five microscopic fields, and all the numbers were in one seminiferous tube, reported as the average of several tubes. Round and dark nuclei with small size located on the ferrous basement membrane are spermatogonia. The largest nuclei in terms of size, the color of the nucleus is paler, and they are located at a distance between the spermatogonia and the center of the tube and are in several rows are spermatocyte cells. The sickle-shaped nuclei with little cytoplasm and are very large in number and located in the center of the tube, and have a tail are spermatids.
3.6. Immunohistochemistry
The samples were washed with PBS in four steps. In order to recover the antigen, hydrochloric acid 2 normal was poured on the samples for 30 minutes. Borate buffer solution was added for five minutes. Samples were washed with PBS. Triton 0.3% was used for 30 minutes to permeabilize the cell membrane. The samples were washed again with PBS. Then, 10% goat serum was added for 30 minutes to block the secondary antibody reaction as background color. Diluted primary buffer (1: 100) with PBS was added to the sample, and after creating the storage environment, it was placed in a refrigerator at 2 - 8 degrees one night to prevent tissue drying. The next day, the cells were washed with PBS four times for five minutes. The secondary antibody was added to the sample with a dilution (1: 150) and then incubated at 37 degrees for 1: 30 minutes in the dark. After that, the sample was transferred from the incubator to the dark room, and after washing four times, DAPI was added to them, the samples were immediately removed, and PBS was added. In the last step, the samples were observed by Olympus fluorescent microscope with a 400 lens to confirm the markers.
3.7. Statistical Analysis
Shapiro-Wilk test was used to investigate the normal distribution. To examine the difference between the healthy and doxorubicin groups, independent sample t-test was used. Afterward, two-way analysis of variance (ANOVA) with Bonferroni’s post hoc test in SPSS 21 was used to measure the effect of interaction between HIIT and Cr (P ≤ 0.05).
4. Results
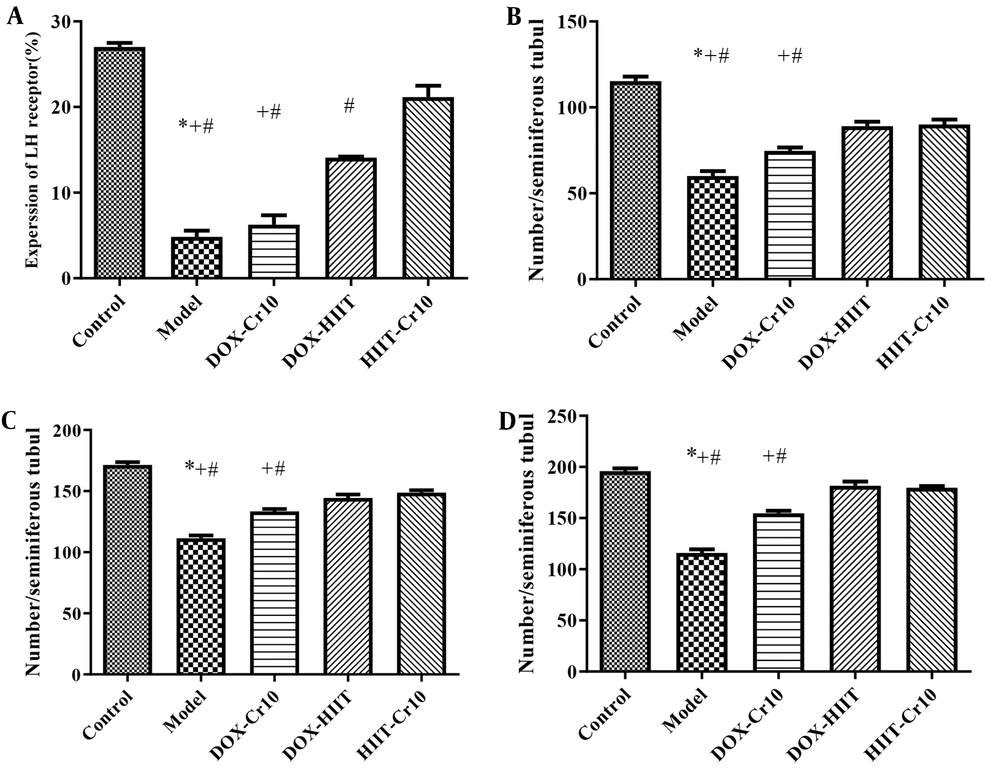
The mean ± standard deviation (SD) cell count of spermatogenesis and levels of LHR expression in the Leydig cells of rats’ testicular tissues are reported in Figure 1. Also, the results of independent samples t-test showed that doxorubicin injection had a significant effect on decreasing spermatogenesis and LHR (P = 0.0001) compared to the control group. The results of two-way ANOVA showed that eight weeks of HIIT or Cr consumption, as well as the interaction of HIIT and Cr consumption, had a significant effect on increasing LHR and spermatogenesis (Table 1).
Effect of aerobic training and crocin (Cr) on lutein hormone receptor (LHR). A, Spermatogonia; B, Spermatocyte; and C, Spermatid; D, in Leydig cells. Control: Healthy group; model: Doxorubicin (DOX) group; DOX-Cr10: DOX + 10 mg/kg body weight (kg.bw) Cr ; DOX HIIT: DOX + high intensity interval training (HIIT); HIIT-Cr10: DOX + HIIT with 10 mg/kg.bw Cr . Values are expressed as mean ± standard deviation (SD). #, Significance differences of groups compared to HIIT-Cr10; +, Significance differences of groups compared to DOX-HIIT; *, Significance differences of groups compared to DOX-Cr10; P < 0.05.
| Parameter | Comparison of the Model-Control | Two Way-ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HIIT | Crocin | Interaction | |||||||||
| t | P | F | P | Effect Size | F | P | Effect Size | F | P | Effect Size | |
| LHR (%) | 25.59 | 0.0001 | 169.66 | 0.0001 | 0.96 | 21.03 | 0.002 | 0.72 | 9.29 | 0.02 | 0.54 |
| Spermatogonia a | 14.23 | 0.0001 | 70.76 | 0.0001 | 0.90 | 8.84 | 0.02 | 0.53 | 6.72 | 0.03 | 0.46 |
| Spermatid a | 18.35 | 0.0001 | 214.45 | 0.0001 | 0.96 | 35.07 | 0.0001 | 0.81 | 43.14 | 0.0001 | 0.84 |
| Spermatocyte a | 18.18 | 0.0001 | 51.79 | 0.0001 | 0.87 | 5.75 | 0.04 | 0.42 | 66.02 | 0.0001 | 0.89 |
Abbreviations: ANOVA, analysis of variance; HIIT, high intensity interval training; LHR, lutein hormone receptor.
a Cell count
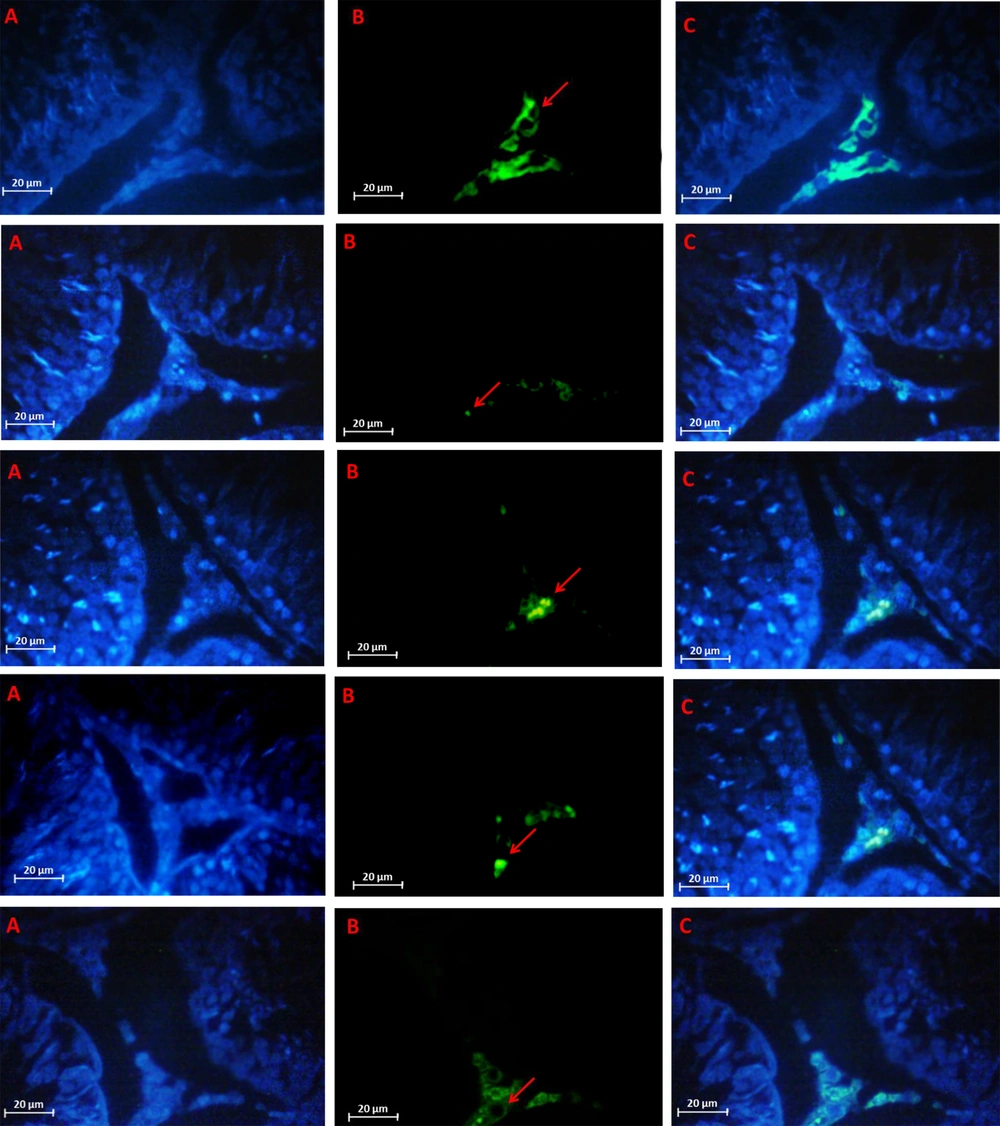
The results of Bonferroni’s post hoc test for LHR changes in Figure 1A showed that in the HIIT + Cr10 group, there was the highest significant increase in LHR levels, and after that, in the DOX + HIIT and DOX + Cr10 groups, respectively, there were the highest increases in LHR levels in doxorubicin-poisoned rats. By IHC method, the highest and lowest expression of growth hormone was shown in Figure 2 in the control and model samples. Further, HIIT + Cr10 caused the highest LH protein expression compared to other interventions.
Luteinizing hormone receptor detected by immunohistochemistry method. A, Nuclei stained by DAPI; B, Primary antibody to LY; C, Merge A&B. In order from top to bottom: Control, model, doxorubicin (DOX) + 10 mg/kg body weight (kg.bw) Cr (Cr10), DOX + high intensity interval training (HIIT), DOX + HIIT + Cr10. Fluorescent microscope (400 × magnification)
The results of Bonferroni’s post hoc test showed that the most favorable effect on changes in spermatogonia (Figure 1B), spermatocytes (Figure 1C), and spermatids (Figure 1D) were exerted by the interaction of training and Cr supplementation. Even though no significant difference was observed between the HIIT + Cr10 and DOX + HIIT groups, the difference between groups with the DOX + Cr10 and the model groups was significant as well. Also, the model group showed a significant difference from other groups regarding the greatest decrease in the number of spermatogonia, spermatocytes, and spermatids (Figure 3).
5. Discussion
The results of the present study showed that consumption of DOX decreased LHR compared to the control group, which indicates the negative effects of DOX treatment on LHR. In line with the decrease in LHR, a significant decrease was also observed in the levels of spermatogonia, spermatocytes, and spermatids. Previous research has also reported the negative effects of DOX treatment on the pathway of spermatogenesis as well as Leydig cells.
In the Cr and HIIT intervention groups, there was a significant increase in LHR expression. In line with an increase in LHR expression, a significant increase in spermatogonia, spermatocytes, and spermatids was also seen, indicating the positive effects of supplementation and training interventions as well as their combination on reducing DOX-induced side effects (1, 15).
Regarding the effect of training on LHR expression and spermatogenesis, Hajizadeh Maleki and Tartibian suggested the benefits of exercise training to prevent or combat testicular dysfunction due to aging, obesity, and DOX treatment (16). Also, Guo et al. reported that proper exercise could improve spermatogenesis in rats and aerobic training mainly increases spermatogenesis by regulating the expression of proteins related to sperm production and differentiation (10).
The positive effects of HIIT are probably the outcomes of reduced oxidative stress and inflammatory status (15, 16). Studies also show that increasing lactate increases endogenous testosterone production by activating the LHR pathway, adenosine 3’: 5’-cyclic monophosphate (cAMP) dependent protein kinase A, steroidogenic acute regulatory protein, and cholesterol side-chain cleavage enzyme P450scc (17). In other words, lactic acid positively regulates the expression of LHR and P450scc genes. During HIIT, blood lactate levels increase from about three mmol in rats to 10 - 15 mmol (18). Therefore, it seems that in this study, the increase in LHR gene expression is associated with the increase in lactate levels due to HIIT, and the positive expression of this gene is associated with increased spermatogenesis during HIIT.
Crocin is one of the active ingredients of saffron, which contains carotenoids that have anti-inflammatory and antioxidant effects on testicular tissue by increasing the total antioxidant capacity (13). The observed positive results of supplementation on DOX-induced side effects may be due to the same antioxidant and inflammatory effects (5-7). Davoodi et al. showed that Cr consumption increases the activity of antioxidants and reduces harmful oxidants in testicular tissue (13). Crocin also decreases the toxicity of parquet and diabetes on testicular tissue in some sperm quality indicators due to its antioxidant and apoptotic properties in rats (19, 20).
Another result of the present study was that the combination of HIIT and Cr consumption reduced the destructive effects of DOX on testicular tissue by increasing LHR expression and spermatogenesis, which was due to the synergistic effect of HIIT and Cr consumption relative to the separate effects of each. Research by Darash et al. also showed that simultaneous use of Cr and continuous aerobic training reduces oxidative and apoptotic factors, but increases antioxidant capacity in testicular tissues (21).
It seems that Cr consumption and training can establish a balance in the cellular defense system against the oxidative state previously known to be induced by DOX in testicular tissues (13, 21). Also, by reducing apoptosis, they provide a condition in favor of the anti-apoptotic system to increase LHR expression and thus spermatogenesis. Nonetheless, the results of this study also showed that the effect of the interaction of HIIT and 10 mg Cr consumption had no significant difference with the effect of training alone, at least on spermatogenesis.
It seems that the combined effects of taking antioxidant supplements such as Cr with physical activity have not been clearly understood so far. Research shows that taking supplements with physical activity affects adaptations resulting from physical activity. Some recent studies have reported that antioxidant supplementation is not always desirable and can stop adaptations resulting from physical activity. However, in this study, the interaction between HIIT and Cr consumption was associated with an increase in spermatogenesis, of which non-significant difference from training alone may be due to the need for more time or dosage, which can be a prospect for future research.
5.1. Conclusions
The clinical findings of this study showed for the first time that testicular tissue toxicity with DOX downregulated LHR expression and decreased the spermatogenesis process. Furthermore, the interaction of training and Cr improves these reducing effects. However, these changes were not recovered to the level of the control group.