1. Background
Breast cancer (BC) is currently the most common cancer among women in Iran and the second leading cause of cancer mortality after lung cancer (1). The Iranian Ministry of Health has documented that one out of eight Iranian women is at risk of BC, accounting for approximately a quarter of all cancers among female patients (2). One of the main distinguishing characteristics of cancer cells is the elevation of the ornithine decarboxylase (ODC1) enzyme, which significantly affects the metabolism of polyamines in cancer cells (3, 4). The ODC enzyme is involved in the first stage of polyamine synthesis and is highly effective in cell growth and DNA synthesis. The lack of this enzyme is associated with cell apoptosis. The ODC activity is abnormally increased in cancer cells.
Several studies on BC have investigated the ODC enzyme function, indicating its role in cancer progression (5). The molecular dysregulation of the ODC1 gene could alter its expression patterns and leads to overexpression in cancer cells. Studies have revealed that ODC downregulation and activity decline could be associated with the reduced growth of cancer cells (6, 7). The current chemical drugs against cancer cells are primarily ineffective and associated with several unwanted side effects. Applying herbal extracts against cancer cells could reduce the side effects of chemical drugs and provide an inexpensive source of treatments.
Rheum khorasanicum is a medicinal plant of the Polygonaceae family. It is endemic to Iran and is morphologically very close to Rheum ribes (8, 9). Numerous studies have been performed on the biological activity of R. ribes and derivative extracts (10-13). Several antibacterial (14, 15) and antioxidant (16) properties have been reported for R. ribes extracts. Moreover, there are some reports on the anti-proliferative and anticancer effects of R. ribes, including research by Tartik et al. who evaluated the cytotoxic effects of R. ribes ethanolic extract on PC3 cells (17). Furthermore, Esmaeilbeig et al. evaluated the apoptotic effects of R. ribes methanolic extract and six other plants on HeLa and K562 cell lines. They reported an IC50 of 144 μg/mL for the methanolic extract of rhubarb in K562 cell lines (18).
Despite studies on R. ribes, the cytotoxic activity of R. khorasanicum has not been previously investigated.
2. Objectives
We aimed to investigate the cytotoxic effects of R. khorasanicum extracts on the MCF-7 BC cell line. We also assessed the mRNA expression of the ODC1 gene in different treatment groups.
3. Methods
3.1. Preparation of Plant Extracts
Rheum khorasanicum was obtained from the Binalood area around Neyshabur city, Iran. The plant was identified by Mr. Taheri from Islamic Azad University, Neyshabur Branch. The voucher specimen (No. 1045) was deposited with the herbarium of Islamic Azad University, Neyshabur Branch, Iran. The extracts were prepared by the Soxhlet extractor in three different solvents, including ethanol, n-hexane, and distilled water. Briefly, the plant stalks were first powdered by grinding, and the extraction was performed for four hours using the Soxhlet method. The solvents were then removed by a rotary apparatus, and the concentrated extracts were dried at room temperature. In this study, the extracts were used at concentrations of 0, 10, 20, 50, 100, and 200 μg/mL, while zero concentration was comprised of deionized distilled water.
3.2. Cell Culture
The MCF-7 breast cancer cell line was obtained from the BuAli (Avicenna) Research Institute, Mashhad University of Medical Sciences, Mashhad, Iran. The DMEM cell culture media, supplemented with 10% FBS (fetal bovine serum) (FBS, Gibco, Canada), was used to culture MCF-7 cells in a T25 tissue culture treated flask at 37°C with 5% CO2 and 95% humidity.
3.3. Cytotoxicity Assay
The MTT assay was used to determine the percentages of viable cells and cytotoxic activity of the extracts. Briefly, cells were plated at a density of 3 × 104 cells/dish in 96-well plates and incubated for 24 h at 37°C. Then, cells were treated with different concentrations of extracts (0, 10, 20, 50, 100, and 200 μg/mL) incubated for 24, 48, and 72 h. A 2 mM MTT solution was added to each well, and plates were incubated for five hours. Then, the supernatant was discarded. The insoluble formazan crystals were dissolved in 200 μL of DMSO solution, and the absorbance was evaluated by an ELISA reader (Awareness, Palm City, FL, USA) at 540 nm. This test was triplicated for each concentration. In order to determine the percentages of viable cells at each concentration of the extract, the mean absorption of treated cells with the desired concentration of the extract was divided by the mean absorption of the equivalent DMSO solution, and the final figure was multiplied by 100. The effective concentration of the cytotoxic extract was reported as IC50, which indicates the concentration at which half of the cells were alive. Prism5.0 software (GraphPad Software Inc., San Diego, CA, USA) was used to calculate the IC50.
3.4. Measuring the Relative Expression of the Ornithine Decarboxylase Gene
In order to assess the ODC1 gene expression level, the treated and untreated cells were subjected to RNA extraction and cDNA synthesis (Parstous, Mashhad, Iran). Quantitative analysis of ODC1 (NCBI accession number: NM-002539.2) gene expression was performed by SYBER Green Real-time PCR using ABI step-one (Applied Biosystems, USA) detection system. Exon-specific primers were designed by Oligo 7.5 software and synthesized by the Nedaye Fan company (Neda Fan Rah, Iran) (Table 1).
| Primer | 5’ > 3’ Sequence | Length (bp) | Tm | GC (%) | Amplicon Size (bp) | Self-Complementarity | Self-3’ Complementarity |
|---|---|---|---|---|---|---|---|
| Ornithine Decarboxylase | 108 | ||||||
| FW | GTGGGTGATTGGATGCTCTTTG | 22 | 59.83 | 50 | 2 | 0 | |
| RV | ACCAGGCTAACTACTCGCTCAA | 22 | 59.03 | 50 | 5 | 1 | |
| ACTB | 154 | ||||||
| FW | TCCATCATGAAGTGTGACGT | 20 | 56.87 | 45 | 6 | 4 | |
| RV | GAGCAATGATCTTGATCTTCAT | 22 | 54.32 | 36.36 | 8 | 4 |
In order to perform qRT-PCR, a cDNA template was constructed using specific primers designed for each gene (beta-actin as internal control). The reaction mixture consisted of 10 μL of 2X SYBER Green PCR Master Mix (Ampliqon, Denmark), 0.4 μL of each primer, 6.2 μL of dH2O, and 3 μL of cDNA. Temperature and cycling conditions included an initial denaturation at 95°C for 10 minutes, then 40 cycles of denaturation at 95°C for 15 seconds, and annealing and elongation at 60°C for 60 seconds. The ΔΔCt method was used to analyze the data obtained from real-time PCR.
3.5. Statistical Analysis
SAS 9.1 software was used to analyze the data variances related to the cytotoxic effects. The Ct values were extracted from real-time PCR, and statistical analyses were performed after calculating the relevant parameters. GraphPad Prism software was used to depict the graphs.
4. Results
4.1. Cytotoxic Effects of Extracts
The results indicated the cytotoxic effects of R. khorasanicum extract at the administered concentrations. We showed that hexane and ethanol extract exerted more cytotoxic effects than the aqueous extract by increasing concentration and time (P ≤ 0.01) (Figure 1). The extracts possessed cytotoxic effects on MCF-7 cancer cells. The extract concentration that reduced the survival to lower than 50% was considered IC50 (Table 2). The lowest IC50 was calculated for the hexane extract, so after 72 hours of treatment of cells with hexane extract, IC50 was 18.3 μg/mL. The ethanolic and aqueous extracts followed it.
| Extract Type | 24 h | 48 h | 72 h |
|---|---|---|---|
| Aqueous | > 300 | 140.1 | 49.3 |
| Ethanol | 267 | 80.7 | 19.6 |
| n-Hexane | 219 | 77.3 | 18.3 |
4.2. Effect of Extract on the Relative Expression of Ornithine Decarboxylase Gene
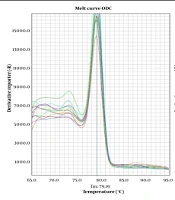
The melting curve diagrams resulting from the qRT-PCR showed the presence of a peak and the absence of additional peaks, indicating the specific amplification of the components and the appropriate efficiency of the PCR (Figure 2).
The changes in ODC1 gene expression in the MCF-7 cell line treated with different concentrations of R. khorasanicum extracts compared to the control indicate a decrease in gene expression by increasing the extract concentrations. Ethanol extract had a more significant effect on ODC1 gene expression than aqueous extract. At the concentration of 50 μg/mL, the ethanolic extract reduced the expression of the ODC1 gene by 1.65 folds, while the aqueous extract reduced the expression of the ODC1 gene by only 1.3 folds (P ≤ 0.01) (Figure 3).
Comparison of changes in ornithine decarboxylase (ODC1) gene expression in MCF-7 cell line treated with different concentrations of extracts. The data are repeated three times per concentration with standard deviation. The means of all data indicate three replicated of a single combination, and ** represents a significant difference at the level of 0.01 based on Duncan’s test.
5. Discussion
The present study showed that R. khorasanicum extracts exerted dose-dependent cytotoxic effects on MCF-7 breast cancer cells. Numerous studies have examined the biological and anti-proliferative activities of rhubarb extracts. Tartik et al. (17) suggested the biological activity of rhubarb root ethanolic extract on PC3 and HUVEC cancer cells and showed that these cells were affected in dose- and time-dependent manners compared to control cells, while their stability was reduced. The study by Shiezadeh et al. (19) examined the toxicity and apoptotic properties of ethyl acetate, hexane, and water extracts of another species of this plant (Rheum turkestanicum) on MCF-7 cancer cells, Hela, and blood lymphocytes, and showed that the hexane and ethyl acetate extracts could reduce malignant cancer cells at the proper dose and time point. Moreover, Uyar et al. (20) showed that rhubarb root’s ethyl acetate extract could possess antioxidant properties. According to the results, R. khorasanicum could be considered a plant with anticancer compounds.
Among all three extracts studied in this study, n-hexane extract showed the highest cytotoxic effects. Due to its low polarity, hexane can extract molecules with a low polarity that usually pass better through cell membranes and have more cytotoxicity (21). Increased levels of the ODC enzyme have an essential effect on the metabolism of polyamines in cancer cells. Polyamines are ubiquitous molecules in eukaryotic cells and play a key role in cell growth, survival, and differentiation (7, 22). Accordingly, identifying them and their synthesis pathways would be crucial in understanding cell biology and, ultimately, a suitable target for controlling cell growth. Various studies have shown that the ornithine decarboxylase enzyme could be a suitable target for chemotherapy. According to the literature, a decrease in the amount and activity of ODC reduces the growth of cancer cells (4, 23). The application of herbal compounds or plant extracts has drawn considerable attention in anticancer research studies, while the reduction of polyamines and ornithine decarboxylase is among the most noticeable alterations. Grimminger et al. showed that the mRNA expression of the ODC1 gene in cancerous tissues was significantly higher than in non-cancerous tissues (24). In another research study by Sharma et al. (25), the Hibiscus rosa extract was administered in the cancerous tissue of mice and prevented the progression of cancer, while the activity of ODC was decreased compared to the control group. In another study, the mice transplanted with human MDA-MB-231 breast cancer cells were fed with grape extract for 33 days. The expression of several genes, including ODC1, was then measured. The results showed a decrease in the expression of the ODC1 gene in treated mice compared to the control group (26).
In this study, after carefully examining the cytotoxicity results obtained from all extracts, aqueous and ethanolic extracts with moderate cytotoxicity were selected to study gene expression. Concentrations less than IC50 were also used. The results showed that the plant extracts could reduce the expression of the ODC1 gene in cancer cells in a dose- and time-dependent manner. Decreased expression of the ornithine decarboxylase 1 gene and consequently decreased concentrations of polyamines can affect the proliferation of cancer cells and reduce the survival of MCF-7 cells.
In conclusion, there are several compounds in R. khorasanicum, such as phenolic compounds as the most important ones with potential anticancer properties. These compounds could be extracted using solvents. The cytotoxicity observed in this study indicates the presence of anticancer compounds in R. khorasanicum. Understanding the exact mechanism of these compounds requires further studies. However, the anticancer effect of R. khorasanicum compounds could be explained due to the reduced expression of the ODC1 gene in this study.