1. Background
Pontocerebellar hypoplasia (PCH) is a group of neurodegenerative disorders with an autosomal recessive inheritance pattern. Such diseases have a prenatal onset and primarily affect the growth and survival of the neurons in the cerebellar cortex. This event leads to microcephaly, seizures, cerebellar hypoplasia, and a significant delay in motor and cognitive development (1-3).
Recent classification divided PCH into sixteen subtypes based on the OMIM database. Although PCH is a severe developmental disorder, clinical and neurological differences may exist between and within different subtypes. For example, PCH1 is usually characterized by the additional dysfunction of motor neurons in the spinal cord, which resembles infantile spinal muscular atrophy. PCH type 2 represents progressive microcephaly along with epilepsy, severe chorea, extrapyramidal dyskinesia, and normal spinal cord signs (4).
Different genes are associated with each subtype of PCH, which may broaden the clinical features. For example, the VRK1, EXOSC3, EXOSC8, EXOSC9, SLC25A46, and EXOSC1 genes have been reported for PCH1. Also, the TSEN54, TSEN2, TSEN34, and SEPSECS genes have been associated with different subtypes of PCH2. The PCH2 subtypes are classified based on the order of identifying responsible genes. One subtype of PCH2 is PCH2B (OMIM: 612389), which occurs by the homozygous or compound heterozygous mutations in the TSEN2 gene (4).
The TSEN2 gene encodes a heterotetrameric enzyme as a subunit of the tRNA splicing endonuclease complex involved in the maturation of tRNA molecules. Other subunits of this complex are encoded by the TSEN15, TSEN34, and TSEN54 genes (4-6). The functional complex catalyzes the tRNA processing through the intron removal, a process required for the proper protein synthesis for cell growth and division (7).
Due to several different genes and the overlapping clinical features of PCH, whole exome sequencing (WES) is considered as a useful method for detecting variants responsible for diseases. Although targeted gene panels have been developed, they are at risk of being outdated due to the increased rate of new gene discoveries (8). Thus, WES seems to be a suitable method of detecting the genetic cause of pontocerebellar hypoplasia.
In the current study, we reported two patients with similar phenotypes attributed to pontocerebellar hypoplasia. WES was used to find the genetic cause of the disorder in the patients’ families living in the province of Khuzestan, Iran. Our results showed that the families shared a common missense mutation in the TSEN2 gene.
2. Objectives
Our study was aimed to find the genetic cause of a disorder in two patients from two families with cerebellar hypoplasia through WES, followed by in silico and segregation analyses.
3. Methods
3.1. Clinical Reports
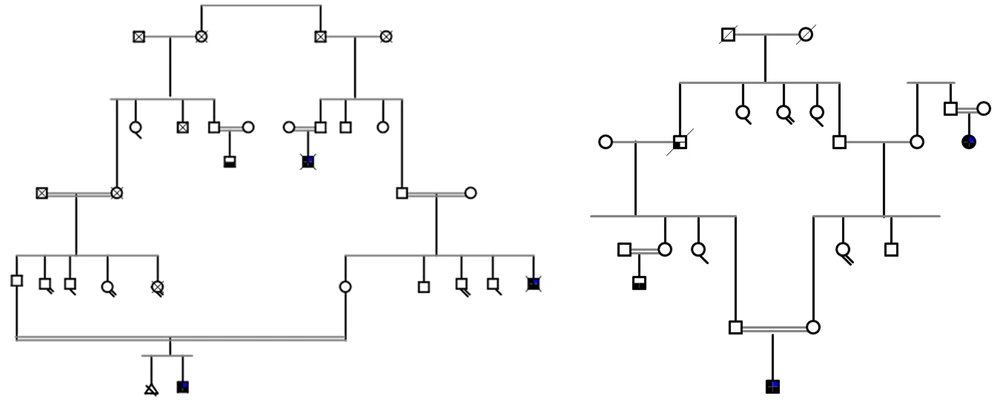
The ethics committee of Shahid Chamran University of Ahvaz, Iran, approved the study (ethical code: EE/99.3.0239631/scu.ac). Two patients with undiagnosed developmental delay were referred for genetic testing. Both had some symptoms related to PCH, such as progressive microcephaly, cerebellar hypoplasia and atrophy, problems in movement, dystonia, and intellectual disability. Other features of dysmorphism were absent in them. Patient I was a 4-years-old boy with healthy consanguineous parents and a history of several deceased affected patients with developmental delay and similar symptoms in the family. The patient had developmental delay, intellectual disability, and such movement disorders as dystonia and spasticity. The patient’s brain MRI demonstrated cerebellar atrophy, while his EEG showed no abnormal epileptic waves. Patient II was a 6-months-old male infant with healthy consanguineous parents. The patient had developmental delay, microcephaly, and hypotonia according to his physical examination. Pedigrees of the two families are shown in Figure 1.
3.2. Karyotyping
Karyotyping was done for the patients according to the previous protocols to check for any chromosome anomalies in them (9, 10).
3.3. DNA Extraction
Blood tubes of 5 mL were collected from the patients and their available family members using EDTA as an anticoagulant. Genomic DNA was isolated using the salting out procedure and stored at -20°C. The DNA quantity was checked using the NanoDrop-1000 spectrophotometer (NanoDropTM 2000 Spectrophotometer, Thermo ScientificTM, USA), and its quality was examined by agarose gel electrophoresis.
3.4. Whole Exome Sequencing
WES for the two extracted DNA samples was done using the commercial service (Macrogen Inc., Seoul, Korea). The exome capture of DNA from the two probands was accomplished using the Agilent SureSelect Target Enrichment Kit (SureSelect_v6 Agilent USA), followed by paired-end sequencing on the Illumina HiSeq4000 system (Illumina Inc., San Diego, CA). FASTQ raw data files were converted to the BAM files format. Then, the Genome Analysis Toolkit (GATK) was applied for calling and filtering the variants. Finally, the variants were filtered based on parameters including frequency, genomic region, zygosity, protein effect, and correlation with the clinical features (11, 12).
3.5. In Silico Analysis of the Variants
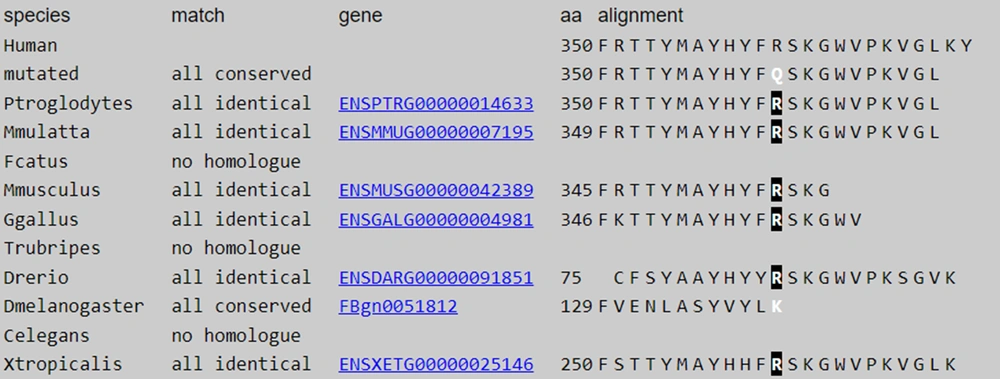
Variants with minor allele frequency (MAF) ≤ 0.01 were filtered for further analysis by applying different databases such as Exome Aggregation Consortium (ExAC), 1000 Genome Project, Genome Aggregation (gnomAD), and Iranome databases (13-16). Then, synonymous and intronic variants were filtered out. Also, heterozygous variants were excluded based on the consanguineous background of the two families. However, the intronic and heterozygous variants in the patients were investigated later. Different in silico tools, such as SIFT, FATHMM, Mutation Taster, Polyphen 2, and CADD, were applied to predict the pathogenicity of the variants. Finally, VarSome was checked to find the pathogenicity of the variants based on the American College of Medical Genetics and Genomics (ACMG) guidelines. The conservation of the desired sequence was investigated through the alignment of the region encompassing the mutation among different species by MultAlin (multalin.toulouse.inra.fr/multalin) (17, 18).
3.6. Bioinformatics Analysis of Protein Stability and Structure
The I-Mutant and Missense3D algorithms were used to predict the functional consequences of the mutation in protein. Changes in the stability of the mutant form of the protein were checked using I-Mutant. This tool, which uses the experimental data of ProTherm, can automatically predict protein stability changes due to mutations. Missense3D predicts the effect of missense mutation on the structure of proteins. The 3D structure of the wild-type protein and the missense variant was derived from this web server.
3.7. Sanger Sequencing and the Segregation Analysis of the Variants
The selected candidate variant (TSEN2: p.R350Q:c.G1049A) was validated by Sanger sequencing in both family members to confirm the segregation of the variants in the families. The polymerase chain reaction (PCR) was performed using primers amplifying the region. The primers (listed in Table 1) were designed with Primer3. ABI BigDye Terminator v3.1 Cycle Sequencing Kit, ABI 3100, and 3500xL Dx Genetic Analyser (Applied Biosystems, Foster City, CA, USA) were used for Sanger sequencing.
| Primer | Sequences | Size of PCR Product |
|---|---|---|
| TSEN2 | 480 bp | |
| Forward | 5′-GACCTTCTTGCTAAATTGCTCTGC-3′ | |
| Reverse | 5′- CTTCTTAGAAAACATCATCAACAGCAC -3′ |
4. Results
4.1. Clinical Findings
Both families were known to be consanguineous from the first cousin marriages (Figure 1). The affected members showed similar symptoms including developmental delay, intellectual disability, hypotonia, and microcephaly. After undergoing a detailed examination by the physicians, the patients were referred for genetic analysis through next-generation sequencing to find probable mutations responsible for the disease.
4.2. Karyotype Analysis
The karyotype study of 20 metaphases showed the "46, XY" normal males. There were no chromosomal changes in either of the patients.
4.3. Analysis of Whole Exome Sequencing Data
The WES data were analyzed for the probands of each family. The data showed that the "mean depth: Coverage: Total number of variants" was (90.9X:97%:291245) and (91.7X:93.5:287456) for patients I and II, respectively. The data were also filtered based on inheritance and allele frequency to identify homozygous pathogenic variants. Finally, WES revealed a novel missense mutation, p.R350Q:c.G1049A, in the TSEN2 gene. Previously known as rs775547616, the variant showed very low frequency in the normal population. Also, the analysis of 800 normal Iranian cases showed no homozygosity in the population (Iranome.ir). Different in silico tools showed that the variant was pathogenic and had destroying effects on the protein structure (Table 2). Also, the alignment at the mutation locus showed that the variant was in a highly conserved region.
| Protein | Amino Acid Change | SIFT | Mutation Taster | MutPred | |||
|---|---|---|---|---|---|---|---|
| Score | Prediction | Accuracy | Prediction | Score | Prediction | ||
| TSEN2 | R350Q | 0 | Damaging | 0.9999 | Disease causing | 0.907 | Pathogenic |
4.4. The Effect of Mutation on Protein Stability and Structure
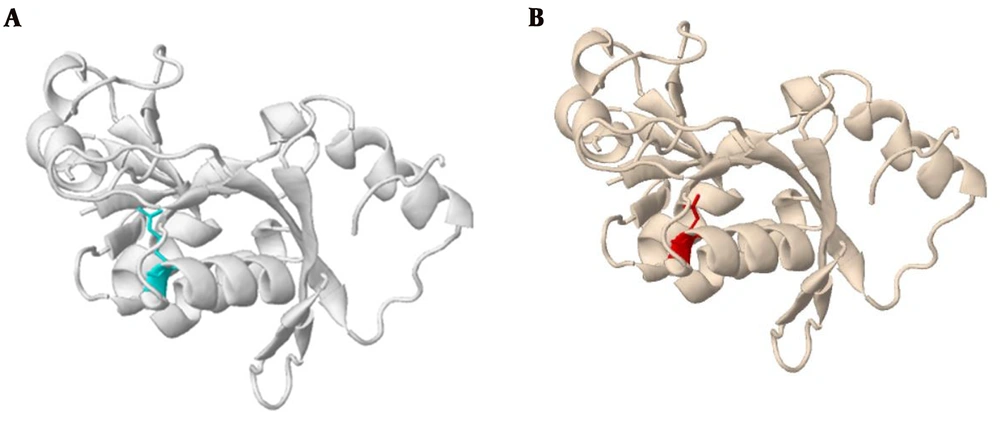
Analyzing the effects of mutation on protein using I-Mutant showed the decreased stability of the protein (shown in Table 3). Moreover, analyzing the protein structure through Missense3D showed structural damage in the mutant form (Figure 2). The result showed that this substitution replaced a buried charged residue (Arg) with an uncharged residue (Gln).
| Position | WT | NEW | Stability | RI | pH | T |
|---|---|---|---|---|---|---|
| 350 | R | Q | Decrease | 8 | 7.0 | 25 |
Abbreviations: WT, amino acid in wild-type protein; NEW, new amino acid after mutation; RI, reliability index; T, temperature in Celsius degrees; pH: -log[H+].
4.5. Sanger Sequencing and Segregation Analysis
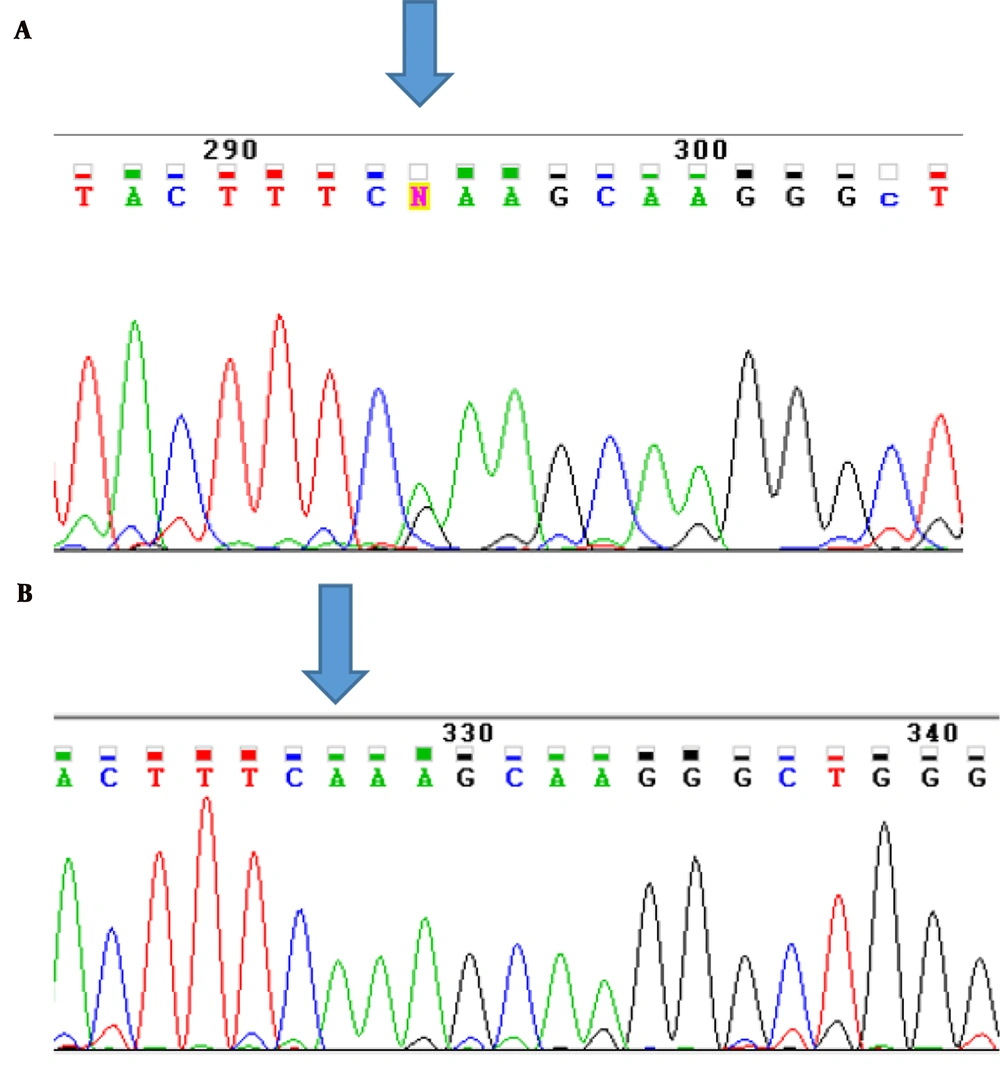
The affected individuals, their parents, and the unaffected individuals from the two families were genotyped by Sanger sequencing. Sanger sequencing results confirmed that the mutation was inherited as homozygous in the two probands, while their parents were heterozygous. However, the healthy family members did not show homozygosity for this variant (Figure 3).
5. Discussion
TSEN2 encodes one of the subunits in the heterotetrameric tRNA splicing endonuclease complex, which is fundamental for the normal processing of tRNAs. Different mutations in this gene have been associated with pontocerebellar hypoplasia type 2 (3, 19). A few mutations have already been recognized in patients with PCH2. The major findings of this disease include microcephaly, developmental delay, and movement problems (6). The TSEN2 gene is located at 3p25.2 and contains 12 exons (transcript ID: ENST00000284995.11) (20).
Up to now, different mutations have been reported in the TSEN2 gene, causing PCH2B. In 2008, Budde et al. identified mutations in TSENs, including TSEN2 Y309C, in the affected pontocerebellar hypoplasia (19). Bierhals et al. detected the novel missense mutation in TSEN2 (p.G312R) (4). Also, Wang et al. reported compound heterozygosity for two pathogenic mutations in TSEN2 (p.Glu302Lys and p.Arg452*). The mutations were associated with PCH type 2B, characterized by abnormalities in the cerebellum and brainstem with extrapyramidal dyskinesia, epilepsy, and progressive microcephaly (21).
In the present study, we found two patients with similar symptoms from two unrelated families in Ahvaz, Southwest Iran. The cases’ parents were both first-cousins. Therefore, WES was used to investigate the cause of genetic mutation in the cases in whom developmental delay, hypotonia, and microcephaly were evident. We conducted next-generation sequencing on both affected probands and then confirmed the variant with different in silico tools, Sanger sequencing, and segregation analysis. The WES analysis revealed a homozygous missense mutation (p.R350Q) in the exon 8 of the TSEN2 gene, associated with pontocerebellar hypoplasia type 2B. This missense mutation, located in a conserved region (Figure 4), was shown to disrupt the normal function of protein through different software.
The known TSEN2 gene mutations causing PCH2B disturb the proper function of the tRNA splicing endonuclease complex. This can impair the production of RNA molecules and the translation of different types of proteins. These events seem to have the most adverse effects on tissues with fast growth, such as the fetal brain. However, the correlation between the reduced function of the tRNA splicing endonuclease complex and the impaired brain development process is unclear. Since rare mutations are reported for this disease, any novel mutation can reveal the correlation between the genotype and phenotype for this disorder and extend our knowledge of this gene.
Based on our bioinformatics analysis and survey of the normal population in different databases, TSEN2:R350Q can be considered as the candidate pathogenic variant identified by WES.