1. Context
At first, statins were known as new antibacterial drugs, and from 1976, it was revealed that this category of drugs is the inhibitors of hydroxymethylglutaryl-CoA (HMG-CoA) reductase. Statins are widely administered to decrease atherosclerosis via reducing plasma levels of low-density lipoprotein cholesterol (LDL-C) in patients with dyslipidemias and coronary heart disease (1, 2). Statin medications consists of Atorvastatin, Simvastatin, Lovastatin, Mevastatin, Pravastatin, Fluvastatin, Cerivastatin, Pitavastatin, and Rosuvastatin (3). The justification for the use of statins in prevention (JUPITER) study indicated that the administration of statins increases insulin resistance in animal models and patients, and approximately enhances 20 - 30% risk of diabetes (2). Type 2 diabetes development caused by statins reduces insulin secretion of islets of Langerhans. However, the mechanism of this event remains unclear, but some studies showed that “statins diminish pancreatic β-cell function via Ca2+ signaling pathways impairment, compromise insulin signaling and down-regulating of insulin-responsive glucose transporter 4 (GLUT-4) (4). It was revealed that elimination of HMG-CoA reductase in a β-cell exhibited hypoinsulinemic hyperglycemia due to reduces in both β-cell mass and insulin secretion (5). One study reported that simvastatin as an (HMG-CoA) reductase inhibitor decreases insulin secretion in MIN6 β-cells through inhibition of acetylcholine receptor activity, free fatty acid receptor 1, and calcium release from intracellular stores (6). Moreover, contradictory results have been obtained regarding the effect of statins on insulin secretion from the islets of Langerhans. Some of them believe that these drugs increase insulin secretion (7) and the others show a reduction in insulin secretion from the islets (8). Therefore based on the effect of statins in the development of diabetes by reducing or increasing insulin secretion, we performed a systematic review of animal, cell, and human studies investigating the effect of statins administration on insulin secretion of islets of Langerhans.
2. Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (9).
The electronic literature search was done in the databases Medline (PubMed), Scopus, Web of Science, and ProQuest.
2.1. Search Strategy
All electronic databases were assessed from 2000/01/15 until 2021/12/15. The search strategy for the PubMed database is exhibited in Table 1, and the other databases were attached to this article as appendices 1 - 3. The search strategy terms were consisted of three elements: (1) statin (Hydroxymethylglutaryl-CoA reductase inhibitors); (2) insulin secretion, and (3) islets of Langerhans, and were used in combination with each other. The search elements were evaluated by the controlled vocabulary terms called Mesh Term system. Limits were used to include only review articles and no limits were set on language.
| Number | Search Syntax | Results |
|---|---|---|
| 1 | ("Hydroxymethylglutaryl-CoA Reductase Inhibitors"[tiab] OR “Hydroxymethylglutaryl CoA Reductase Inhibitors”[tiab] OR (Inhibitors[tiab] AND “Hydroxymethylglutaryl-CoA Reductase”[tiab]) OR (“Reductase Inhibitors”[tiab] AND Hydroxymethylglutaryl-CoA[tiab]) OR “HMG-CoA Reductase Inhibitor”[tiab] OR “HMG CoA Reductase Inhibitor”[tiab] OR Statin[tiab] OR (Statins[tiab] AND HMG-CoA [tiab]) OR “HMG-CoA Statins”[tiab] OR (Statins[tiab] AND “HMG CoA”[tiab]) OR (Inhibitors[tiab] AND “HMG-CoA Reductase”[tiab]) OR (Inhibitors[tiab] AND “HMG CoA Reductase”[tiab]) OR (“Reductase Inhibitors”[tiab] AND HMG-CoA [tiab]) OR “HMG-CoA Reductase Inhibitors”[tiab] OR “HMG CoA Reductase Inhibitors”[tiab] OR (Inhibitors[tiab] AND “Hydroxymethylglutaryl-Coenzyme A”[tiab]) OR “Hydroxymethylglutaryl-Coenzyme A Inhibitors”[tiab] OR (Inhibitors[tiab] AND “Hydroxymethylglutaryl Coenzyme A”[tiab]) OR (Inhibitors[tiab] AND Hydroxymethylglutaryl-CoA[tiab]) OR “Hydroxymethylglutaryl-CoA Inhibitors”[tiab] OR (Inhibitors[tiab] AND “Hydroxymethylglutaryl CoA”[tiab]) OR “Hydroxymethylglutaryl-CoA Reductase Inhibitor”[tiab] OR “Hydroxymethylglutaryl CoA Reductase Inhibitor”[tiab] OR (“Reductase Inhibitor”[tiab] AND Hydroxymethylglutaryl-CoA[tiab]) OR Statins[tiab]) | 45108 |
| 2 | (“insulin secretion”[tiab] OR (secretion[tiab] AND insulin[tiab])) | 49646 |
| 3 | (“Islets of Langerhans”[tiab] OR “Langerhans Islets”[tiab] OR “Pancreatic Islets”[tiab] OR (Islet[tiab] AND Pancreatic[tiab]) OR (Islets[tiab] AND Pancreatic[tiab]) OR “Pancreatic Islet”[tiab] OR (Pancreas[tiab] AND Endocrine[tiab]) OR “Endocrine Pancreas”[tiab] OR “Islet Cells”[tiab] OR (Cell[tiab] AND Islet[tiab]) OR (Cells[tiab] AND Islet[tiab]) OR “Islet Cell”[tiab] OR “Islands of Langerhans”[tiab] OR “Langerhans Islands”[tiab] OR Nesidioblasts[tiab] OR Nesidioblast[tiab]) | 51520 |
| 4 | 2000/01/15:2021/12/15[dp] | - |
| 5 | 1 AND 2 | 144 |
| 6 | 2 AND 3 | 11777 |
| 7 | 1 AND 3 | 47 |
| 8 | 1 AND 2 AND 3 AND 4 | 21 |
The Search Strategy Used in MEDLINE via PubMed
2.2. Inclusion Criteria
Type of publication (article, book, and pepper conference), all language, and type of study (original studies) performed from 2000/01/15 until 2021/12/15 in each country and examines the relationship between Statin, Insulin secretion, and Islets of Langerhans were addressed as inclusion criteria in the present study.
2.3. Article Selection
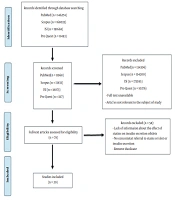
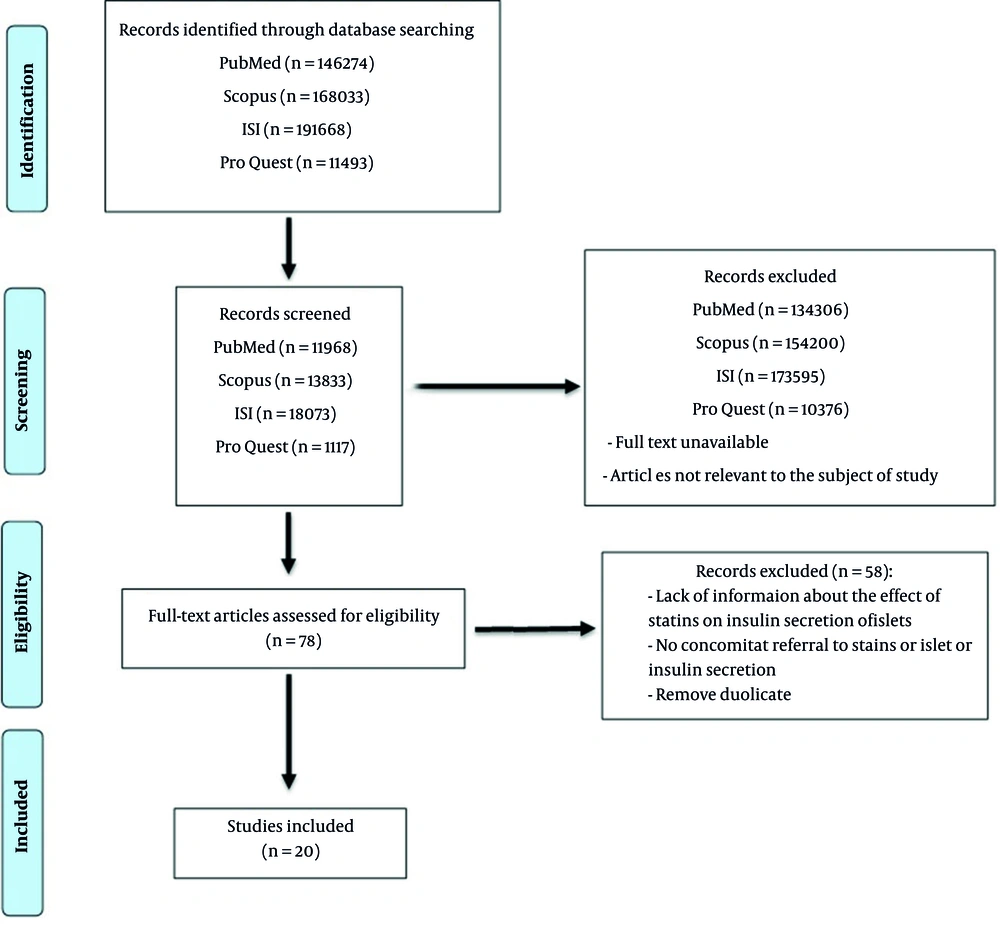
All of the articles were transferred to EndNote (X8) software in the search phase. At the screening stage, the researcher independently reviewed the title and abstract of the articles that fulfilled the selection criteria. During this phase of the study, two independent authors have assessed the selected article (according to the PRISMA flowchart) to ensure that they satisfied the inclusion criteria, and the studies that did not comply with the research objectives was excluded (Figure 1).
3. Data Extraction
Data were extracted according to a form that was created before the literature search. The details of extracted studies were including the First author’s name, publication year, sample size, type of sampling, study population, aims of the publication, experimental methodology (in vivo, ex vivo, and in vitro), key finding, language, gender, age, type of study (Table 2).
| No. | Author | Publication Year | Sample Size | Study Population | Aims | Experimental Methodology | Key Finding | Language | Gender | Age | Type of Sampling | Type of Study |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abe et al. (10) | 2010 | 24 | Mouse & Islet cell | 1. Examine the transport of pravastatin into the islets or the effect of this drug on insulin secretion | In vivo & in vitro | 1. The uptake of pravastatin into β-cells via organic anion transporters contributes to insulin secretion | English | Male | 6 weeks | Random | Original article |
| 2 | Beltowski et al. (11) | 2018 | Not mentioned | Rat & Islet cell | 1. Assessment the effect of statins on insulin secretion and the underlying mechanism | In vivo & ex vivo | 1. Lipophilic statins inhibit glucose-induced insulin secretion by augmenting H-2S signaling in pancreatic islets. 2. The effect is mediated by statin-induced CoQ depletion and may contribute to detrimental effect of statins on glucose homeostasis | English | Male | Not mentioned | Random | Congress |
| 3 | Chang et al. (12) | 2011 | Not mentioned | Islet cell of Rat | 1. Evaluation of the inhibitory effect of statins on glucose-stimulated insulin secretion (GSIS) of pancreatic islet in rat and explore its mechanisms | In vitro | 1. Atorvastatin and fluvastatin may inhibit GSIS by decreasing ATP content in pancreatic islets. 2. This inhibitory effect is related to the strength of its lipophilicity. | Chinese | Not mentioned | Not mentioned | Random | Original article |
| 4 | Hoffmeister et al. (13) | 2020 | Not mentioned | Islet cell of Mouse | 1. assessment of the negative effects of statins on glycemic control and β-cells signaling | In vitro | 1. The diabetogenic risk of statins is coupled to the activity of farnesoid X receptor (FXR) dependent signaling pathways in β-cells. 2. statins abolish the insulinotropic effects of bile acids 3. FXR determines the level of impairment of islet function by the statin | English | Male & Female | 6 - 12 months | Random | Original article |
| 5 | Huang et al. (14) | 2006 | 32 | Rat | 1. Statins influence insulin secretion in vivo in rats through effects on islet blood perfusion | In vivo | 1. The antidiabetic actions of statins and RAS inhibitors might in part occur through the beneficial direct effect on islet insulin secretion. | English | Male | Not mentioned | Random | Original article |
| 6 | Huang et al. (7) | 2007 | 32 | Rat | 1. Investigation of the influence of captopril, irbesartan and pravastatin treatment on pancreatic blood flow, islet blood flow, glycaemia and insulin concentrations in normal female rats. | In vivo | 1. Statins and renin–angiotensin system inhibitors have the antidiabetic actions through the beneficial direct islet effects. | English | Female | Not mentioned | Random | Original article |
| 7 | Huang et al. (15) | 2008 | 50 | Rat | 1. Investigation of the gender-specific effects of ACE inhibition, AngII receptor antagonism, statin treatment and palmitate administration on pancreatic islet blood flow, insulin levels and glycaemia in GK (Goto-Kakizaki) rats | In vivo | 1. A local pancreatic RAS (renin–angiotensin system) and pravastatin may be selectively influencing the pancreatic microcirculation and therefore affecting insulin secretion and glycaemia. 2. NEFAs (non-esterified fatty acids) impaired pancreatic islet blood flow, suppressed insulin secretion and increased blood glucose | English | Male & Female | Not mentioned | Random | Original article |
| 8 | Ishikawa et al. (16) | 2006 | 48 | Islet cell of Mouse | 1. To estimate the direct effects of statins on insulin secretion from pancreatic β-cells, MIN6 cells were treated with pravastatin, simvastatin, or atorvastatin. | In vitro | 1. Glucose-stimulated insulin secretion of islets isolated from C57BL/6 mice was not significantly changed by any of the statins. 2. High doses of lipophilic statins can decrease insulin secretion through either Hydroxymethylglutaryl-CoA reductase inhibitors (HMG-CoA) inhibition or cytotoxicity. | English | Not mentioned | Not mentioned | Random | Original article |
| 9 | Lorza-Gil et al. (17) | 2019 | 32 | Mouse & Islet cell | 1. The diabetogenic effects of pravastatin could be counteracted by treatment with the antioxidant coenzyme Q 10 | In vivo & In vitro | 1. Statins impair β-cell redox balance, function and viability. 2. CoQ 10 supplementation can protect the statins detrimental effects on the endocrine pancreas. | English | Female | 4 weeks | Random | Original article |
| 10 | Lorza-Gil et al. (18) | 2019 | 36 | Mouse & Myotube cell | 1. Long-term pravastatin effects on glucose homeostasis, insulin sensitivity, muscle protein turnover and cell viability. | In vivo & in vitro | 1. In addition to reduced insulin secretion, long-term pravastatin treatment induces insulin resistance and muscle wasting. 2. The diabetogenic effect of statins is linked to the appearance of myotoxicity induced by oxidative stress, impaired insulin signalling, proteolysis and apoptosis. | English | Female | 4 weeks | Random | Original article |
| 11 | Lorza-Gil et al. (19) | 2016 | 20 | Mouse & Islet cell | 1. Treatment of LDLr-/- mice with the HMGCoA reductase inhibitor pravastatin would improve glucose-stimulated insulin secretion. | In vivo & Ex vivo | 1. These results indicate that chronic treatment with pravastatin impairs the insulin exocytosis machinery and increases β-cell death. 2. Prolonged use of statins may have a diabetogenic effect. | English | Female | 4 weeks | Random | Original article |
| 12 | Mizukami et al. (20) | 2012 | 52 | Rat | 1. To examine the effects of high fat diet (HFD) on the islet in GK rats, non-obese type 2 diabetic model 2. To explore if pitavastatin treatment influences the change | In vivo | 1. Pitavastatin treatment improved the HFD-induced islet pathology, and pancreatic insulin contents paralleled the structural changes 2. HFD feeding worsened the islet pathology in GK rats which was suppressed by pitavastatin treatment | English | Male | 4 weeks | Random | Original article |
| 13 | Real et al. (21) | 2018 | 9 | Islet cell of mouse | 1. To examine the relationship between drug lipophilicity (P) and IC50 for KATP block and explore if the IC50's of statins could be predicted from their lipophilicity and whether this would allow one to forecast their acute action on insulin secretion. | In vitro | 1. Although the IC50 for the block of KATP by simvastatin was predicted, the difference between this and therapeutic levels, as well as serum sequestration, explains why hypoglycaemia is unlikely to be observed with acute use of this statin. | English | Male | 3 - 6 months | Random | Original article |
| 14 | Salunkhe et al. (22) | 2016 | 208 | Mouse & Islet cell | 1. To identified the integrated role of rosuvastatin on glucose homeostasis and aimed to understand the cellular mechanisms by which rosuvastatin acts on insulin secretion and glucose uptake. | In vivo & in vitro | 1. Rosuvastatin reduces blood glucose through improved insulin sensitivity. 2. Rosuvastatin have deleterious effects on the β-cells in vitro that may be detrimental in the end. 3. Long term perspective an impaired β-cells function, with reduced insulin content and disturbed Ca2+ signaling, will accelerate the risk of developing hyperglycemia. | English | Female | 8 weeks | Random | Original article |
| 15 | Scattolini et al. (23) | 2016 | Not mentioned | Islet cell of mouse | 1.To evaluate the effects of simvastatin on insulin secretion from single murine islet | In vitro | 1. The data demonstrated that a statin inhibits insulin secretion in intact islets and the single islets respond differently from cell lines on a short time scale. | English | Not mentioned | Not mentioned | Random | Original article |
| 16 | Shen et al. (24) | 2020 | 45 | Mouse & Islet cell | 1. Both in vivo and in vitro approaches to characterize β-cell defects and investigate transcriptome changes in mouse islets after long-term exposure to atorvastatin (ator) were assessed. | In vivo & in vitro | 1. It was demonstrated that ator treatment reduced HMG-CoA reductase-related isoprenoid production in pancreatic islets and hence impaired b-cell mechanistic target of rapamycin (mTOR) signaling and functional mass, thus inducing diabetes | English | Male | 8 weeks | Random | Original article |
| 17 | Takei et al. (5) | 2020 | 19 | Mouse & Islet cell | 1. To investigate the role of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) in the development of beta-cells and glucose homeostasis. | In vivo & In vitro | 1. Deletion of HMGCR in β-cells caused overt diabetes as early as P9 in mice by markedly reducing β-cell mass as well as insulin secretion from each islet. 2. The β-cell mass reduction was mainly caused by impaired proliferation of β-cell immediately after birth, and trans differentiation of β-cells to α-cells might contribute. | English | Male | 5 weeks | Random | Original article |
| 18 | Urbano et al. (25) | 2017 | 36 & 40 | Islet cell of Human & Rat | 1. To explore the effect of a chronic treatment with lipophilic and hydrophilic statins on beta-cell function, using human pancreatic islets and rat insulin-secreting INS-1 cells. | In vitro | 1. The data of study demonstrate that mitochondrial oxidative stress is a key element in the pathogenesis of statin-related diabetes and may have clinical relevance to design strategies for prevention or reduction of statin induced beta-cell dysfunction and diabetes in patients treated with lipophilic statins. | English | Not mentioned | Not mentioned | Random | Original article |
| 19 | Zhao and Zhao (8) | 2015 | 48 | Islet cell of Human | 1. To determine the effect of different statins on the induction of diabetes mellitus. | In vitro | 1. Statins similar but different degree of effects on pancreas islet β cells damage and induce insulin resistance in human skeletal muscle cells (HSkMCs). | English | Not mentioned | Not mentioned | Random | Original article |
| 20 | Zhu et al. (26) | 2019 | 180 | Islet cell of Rat | 1. It was investigated whether pioglitazone can ameliorate insulin secretion and synthesis dysfunction induced by atorvastatin mediated by the upregulation of Free fatty acid receptor 1 (FFA1) expression. | In vitro | 1. Free fatty acid receptor 1 (FFA1) may mediate the atorvastatin-induced pancreatic β-cell dysfunction and pioglitazone may ameliorate this deleterious effect. 2. Pioglitazone may restore insulin secretion and synthesis dysfunction induced by atorvastatin through the upregulation of FFA1 expression. | English | Not mentioned | Not mentioned | Random | Original article |
The Characteristics of Eligible and Remained Studies
3.1. Gathering, Summarizing, and Declaration of Results
Based on the extracted publications, the increasing or decreasing effects of statins/HMG-CoA reductase inhibitors such as Lovastatin, Fluvastatin, Pravastatin, Rosuvastatin, Atorvastatin, and Pitavastatin on insulin secretion of pancreatic islet cells was reported. Then the results were reported using different tables.
3.2. Quality Assessment
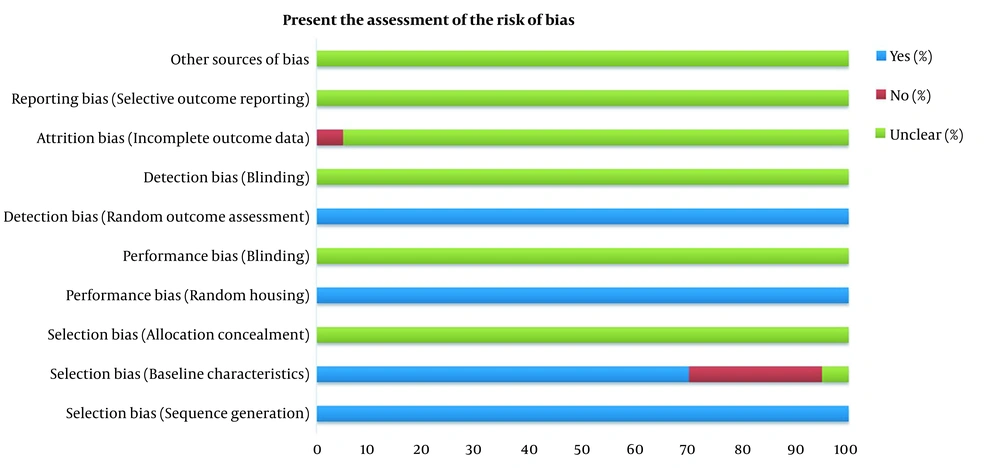
Two authors have independently assessed the risk of bias based on the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias tool (27). This evaluation tool is derived from Cochrane’s Risk of Bias tool for clinical studies that applied to animal studies (28). The tool consists of 10 main questions related to selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. If the answer of questions was "yes" or "no" or "unclear", the question was answered adequately, not answered, and not enough information to answer yes or no respectively. Then according to the received answers, the risk of bias domains was classified as low, high, or unclear.
4. Results
4.1. Search Results
At first, records identified through PubMed, Scopus, ISI, and ProQuest database searching were (n = 146274, n = 168033, n = 191668, and n = 11493 respectively. Then, 44991 studies were remaining for all mentioned databases in the screening stage. After removing full-text unavailable articles and the articles not relevant to the subject of study 78 studies were selected in the eligibility stage of searching. In the end, 20 articles that fulfilled the inclusion criteria of the present study were included and 58 studies were excluded because of the lack of information about the effect of statins on insulin secretion of islets, no concomitant referral to statins or islet or insulin secretion, and removed duplicate (Figure 1).
4.2. Study Characteristics
Ninety-five percent of the studies were articles and 5% were conference. All of the studies were original and experimental. The language of 95% of studies was English and 5% were Chinese. The year of publication of 25% of studies was conducted from 2000 to 2010 and 75% from 2011 to 2021. The population sampling of 20 studies was from mouse and rat or pancreatic islet of these animals, and 2 studies sampling were done in the pancreatic islet of humans. The gender of the administered mice in the included studies was 18.2% female, 22.7% male, and 9.1% not mentioned. This variable was 9.1% female, 18.1% male, and 13.7% not mentioned for the administered rat. Gender was not mentioned in the human study. The sample size of 60% of studies was ≤ 50, 20% was > 50, and 20% was not mentioned. The average age was under 2 months in 40%, over 2 months in 10%, and not mentioned in 45% of animal studies. In addition, this variable was not mentioned in 5% of human studies. Finally, the type of sampling was randomly in all included studies (Table 3).
| Characteristics of Article | No. (%) |
|---|---|
| Content type of studies | |
| Article | 19 (25) |
| Conference | 1 (5) |
| Type of article | |
| Original research and experimental study | 20 (100) |
| Year of publication | |
| 2000 - 2010 | 5 (25) |
| 2011 - 2021 | 15 (75) |
| Population | |
| Animal | |
| Mouse or Islet cell of mouse | |
| Female | 4 (18.2) |
| Male | 5 (22.7) |
| Not mentioned | 2 (9.1) |
| Rat or Islet cell of rat | |
| Female | 2 (9.1) |
| Male | 4 (18.1) |
| Not mentioned | 3 (13.7) |
| Human | |
| Gender | |
| Not mentioned | 2 (9.1) |
| Sample size | |
| ≤ 50 | 12 (60) |
| > 50 | 4 (20) |
| Not mentioned | 4 (20) |
| Average of age | |
| Human | |
| Not mentioned | 1 (5) |
| Animal | |
| Under 2 months | 8 (40) |
| Over 2 months | 2 (10) |
| Not mentioned | 9 (45) |
| Type of sampling | |
| Random sampling | 20 (100) |
Characteristics of Article
4.3. Risk of Bias
The assessment of articles bias and the answer to all questions of the SYRCLE risk of bias tool has been shown in Figure 2. According to our evaluation, all studies had a score that revealed a low risk of bias.
4.4. Statins Administration and Islets Insulin Secretion
As shown in Table 4, however, pravastatin 200 mg/kg (4 weeks; daily; oral) (10) and 0.5 mg/kg (10 seconds; IV) increased islet insulin secretion (7, 14, 15), but 2, 3, and 10-month administration of pravastatin 400 mg/L in drinking water decreased this variable (17-19). In vitro utilization of this statin at the dose of 1000 µM (48 h) (10) increases islet insulin secretion. Moreover, adverse effect was observed after pravastatin 9.4 nM (24 and 48 h) (16), 70 µM (48 h) (17), and 100 nM (24 h) (8) administration in the medium containing islets of Langerhans. Atorvastatin 10 mg/kg (4 month; daily; oral) decreased islets insulin secretion (24). The same effect was observed after in vitro atorvastatin 100 µM (24 h) (12), 15 µM (24 h) (13), 1.9 nM (24 and 48 h) (16), 10 µM (24 h) (24), 10 and 100 ng/mL (24 and 48 h) (25), 100 nM (24 h) (8), and 0.2, 2, and 20 µM (24 h) (26) consumption. In vivo results of rosuvastatin 2 mg/kg (12 weeks) administration in drinking water showed a reducing effect in islets insulin secretion of normal diet (ND) consumed mice, and increased this variable in the high-fat diet group (22). Moreover, rosuvastatin at the doses of 10 µM and 100 nM (24 h) (8, 24) decreased insulin secretion of pancreatic islet cells. A similar effect occurred after fluvastatin 10 (24) and 100 µM (12) was added in the medium containing islets of Langerhans for 24 h. Simvastatin 2.7 nM (24 and 48 h) (16) and 1 µM (about 200 seconds) (23) reduced the secretion of insulin from the islets. Furthermore, this antidiabetic hormone increased after 1 h administration of simvastatin 1 µM or 100 nM in the islets medium (21). However oral ingestion of pitavastatin 3 mg/kg for 16 weeks did not affect on islets insulin secretion (20), but in vitro utilization of this drug at the doses of 100 nM for 24 h decreased this factor (8). In the end, lovastatin 10 µM (24 h) reduced this hormone secretion from the islet cells of the pancreas (24).
| No. | Author | Year | Drug's Name | Dose | Duration | Times of Administration | Type of Prescription | Effect on Insulin Secretion |
|---|---|---|---|---|---|---|---|---|
| 1 | Abe et al. (10) | 2010 | Pravastatin | 200 mg/kg | 4 weeks | Daily | Oral | Increase |
| 1000 µM | 48 hours | Once | Medium | |||||
| 2 | Beltowski et al. (11) | 2018 | Pravastatin | Not mentioned | 1 week | Daily | Oral | No effect |
| Rosuvastatin | ||||||||
| Atorvastatin | Once | Medium | Decrease | |||||
| Fluvastatin | ||||||||
| 3 | Chang et al. (12) | 2011 | Atorvastatin | 100 µM | 24 hours | Once | Medium | Decrease |
| Fluvastatin | ||||||||
| Pravastatin | No effect | |||||||
| 4 | Hoffmeister et al. (13) | 2020 | Atorvastatin | 15 µM | 24 hours | Once | Medium | Decrease |
| 5 | Huang et al. (14) | 2006 | Pravastatin | 0.5 mg/kg | 10 seconds | Once | Intravenous | Increase |
| 6 | Huang et al. (7) | 2007 | ||||||
| 7 | Huang et al. (15) | 2008 | ||||||
| 8 | Ishikawa et al. (16) | 2006 | Pravastatin | 9.4 nM | 24 hours | Once | Medium | No effect |
| Atorvastatin | 1.9 nM | 48 hours | Decrease | |||||
| Simvastatin | 2.7 nM | |||||||
| 9 | Lorza-Gil et al. (17) | 2019 | Pravastatin | 400 mg/L | 2 months | Drinking water | Oral | Decrease |
| 70 µM | 48 hours | Once | Medium | |||||
| 10 | Lorza-Gil et al. (18) | 2019 | Pravastatin | 400 mg/L | 10 months | Drinking water | Oral | Decrease |
| 11 | Lorza-Gil et al. (19) | 2016 | 400 mg/L | 3 months | Drinking water | Oral | Decrease | |
| 12 | Mizukami et al. (20) | 2012 | Pitavastatin | 3 mg/kg | 16 weeks | Daily | Oral | No effect |
| 13 | Real et al. (21) | 2018 | Simvastatin | 1 µM | 1 hour | Once | Medium | No effect |
| Pravastatin | 100 nM | |||||||
| 14 | Salunkhe et al. (22) | 2016 | Rosuvastatin | 0.2 mg/kg | 12 weeks | Drinking water | Oral | Increase in HFD; Decrease in ND |
| 15 | Scattolini et al. (23) | 2016 | Simvastatin | 1 µM | 200 seconds | Once | Medium | Decrease |
| 16 | Shen et al. (24) | 2020 | Rosuvastatin | 10 µM | 24 hours | Once | Medium | Decrease |
| Lovastatin | ||||||||
| Fluvastatin | ||||||||
| Atorvastatin | 10 µM | 24 hours | Once | Medium | ||||
| 10 mg/kg | 4 months | Daily | Oral | |||||
| 17 | Takei et al. (5) | 2020 | Floxed HMGCR | Not mentioned | Decrease | |||
| 18 | Urbano et al. (25) | 2017 | Atorvastatin | 10 ng/mL | 24 hours | Once | Medium | Decrease |
| Pravastatin | 100 ng/mL | 48 hours | No effect | |||||
| 19 | Zhao and Zhao (8) | 2015 | Pravastatin | 100 nM | 24 hours | Once | Medium | Decrease |
| Rosuvastatin | ||||||||
| Atorvastatin | ||||||||
| Pitavastatin | ||||||||
| 20 | Zhu et al. (26) | 2019 | Atorvastatin | 0.2 µM, 2 µM, 20 µM | 24 hours | Once | Medium | Decrease |
The Properties of Statins Administration in the Eligible and Remained Studies
5. Discussion
The main finding of in vivo studies revealed that pravastatin (7, 10, 14, 15, 17-19), and rosuvastatin (22) could increase and decrease islets insulin secretion or concentration, while atorvastatin (11, 24) and fluvastatin (11) just reduced this variable and pitavastatin had no effect on it (20). The results of in vitro studies showed that pravastatin and induced both increasing (10) and decreasing (8, 17) effects on insulin secretion from the pancreatic islet cells. In addition, administration of atorvastatin (8, 11-13, 16, 24-26), rosuvastatin (8, 24), fluvastatin (11, 12, 24), pitavastatin (8), simvastatin (16, 23), and lovastatin (24) decreased islet insulin secretion in the medium.
Abe et al. revealed that the uptake of pravastatin into β-cell through organic anion transporting polypeptides (oatp) is the main reason for increased islet insulin secretion, and the evidence showed oatp3 mediate this transporting (10). However, previous studies elucidated that both oatp1 and oatp2 contributed to this drug membrane transporting (29, 30). The results of Huang et al. study demonstrated a similar effect on the islets insulin secretion and serum insulin level, and the main expressed mechanisms of this event were an increase in the islet blood flow (15). This alteration was occurred via the increase of antithrombotic and anti-inflammatory effects of this statin that induce endothelium-dependent vasodilation (7, 14). In contrast with Abe et al. and Huang et al. studies, Lorza-Gil et al. showed that pravastatin induced hyperglycemia and hypoinsulinemia and decreased glucose‐stimulated pancreatic islet insulin secretion (17). These effects may be related to the increased lipid peroxidation and overproduction of free radicals such as hydrogen peroxide (H2O2) which leads to islet oxidative stress damage including impaired insulin signaling, proteolysis, apoptosis, and cell death (18, 19). Consequently, chronic exposure to pravastatin reduced the production of coenzyme Q10 (CoQ10) is a major intracellular antioxidant in the liver and this alteration can induce oxidative stress in the pancreatic islets. Therefore, in vivo, ex vivo, and in vitro utilization of CoQ10 supplementation improved statin-induced hyperglycemia, insulin resistance, glucose-stimulated insulin secretion (GSIS), and β-cell apoptosis through the elimination of oxidative stress (17).
Shen et al. showed that chronic treatment of high-fat diet (HFD) mice with atorvastatin, lovastatin, rosuvastatin, and fluvastatin decreased β-cell mass and size, quantities of mature insulin granules, and impaired glucose-induced insulin secretion (GIIS). Atorvastatin induced these disorders via the impairment of isoprenoid production, the expression of small G proteins, and the mechanistic target of rapamycin (mTOR) signaling as key pancreatic transcription factors (TFs). Moreover, Geranylgeranyl pyrophosphate supplementation recovered these alterations and improved the negative effects of atorvastatin on β-cell function (24). Urbano et al. confirmed that administration of atorvastatin as a lipophilic statin inhibits GIIS in pancreatic human islets and rat insulin-secreting cells (INS-1), and this effect was related to the mitochondrial dysfunctions induced by oxidative stress, reactive oxygen species (ROS) overproduction, and suppressed CoQ10 as an antioxidant produced by the liver (25). Moreover, the islets incubation with atorvastatin and fluvastatin 100 μM indicated a significant decrease in ATP content and GSIS them. Therefore, these statins may inhibit GSIS via reducing ATP content and strength of their lipophilicity in the islet of Langerhans (12). Chenodeoxycholic acid (CDC), as a bile acid, can increase insulin secretion through elevation of Intracellular Ca2+ and farnesoid X receptor (FXR) as a member of the nuclear receptor superfamily with an important activity in the lipid and glucose metabolism. It was reported that atorvastatin reduced GSIS without affecting ATP synthesis in cultured islets. This effect was occurred by inhibition of CDC and FXR activities in the β-cell. Finally, these disorders may play a key role in the progression of diabetes mellitus (13, 31). Free fatty acid receptor 1 (FFA1) participates in the GSIS, and atorvastatin diminished potassium-stimulated insulin secretion through inhibition of FFA1 and pancreatic and duodenal homeobox 1 (PDX-1) in INS-1 cells. Hence, FFA1 regulates the atorvastatin-induced pancreatic β-cell dysfunction and pioglitazone improved this disorder by the increase of FFA1 expression (26). Human pancreatic β-cells exposure to 100 nM statins including atorvastatin, pravastatin, rosuvastatin, and pitavastatin decreased cell viability, rate of insulin secretion, and GIIS (28 mM). Also, atorvastatin and pravastatin reduced glucose transporter 2 (GLUT-2) expressions, and atorvastatin, pravastatin, and rosuvastatin inhibited GLUT-4 levels in human skeletal muscle cells (HSkMC). Therefore, statins in addition to pancreatic β-cells damage, Statins can induce insulin resistance in HSkMC. However, 100 nM of mentioned statins induced β-cells dysfunction, but these effects were not observed at doses of 10 and 1 nM (8). Beltowski et al. showed that atorvastatin or fluvastatin reduced GIIS during in vivo and ex vivo situations. The production of signaling endogenous hydrogen sulfide (H2S) that inhibits islets insulin secretion can be affected by statins. Hydrogen sulfide (H2S) production was more in the rats’ islets of Langerhans that received atorvastatin or fluvastatin. Hence, these statins inhibit GIIS via augmenting H2S signaling in the isolated islets (11).
Rosuvastatin administration showed two different effects in HFD and ND mice. This statin reduced insulin content in ND and HFD mice, while the insulin secretion process amplifies in HFD ingested group. Also, deleterious effects such as impaired β-cell function, decreased insulin content, disturbed Ca2+ signaling were observed in long-term consumption of rosuvastatin. Thus, this drug can accelerate the risk of new-onset diabetes through a negative effect on β-cells (22).
As revealed in Mizukami et al. study, Pitavastatin treatment did not increase islet insulin content in normal GK and Wistar rats. However, this statin suppressed hepatic lipid contents, islet fibrosis, and macrophage migration in HFD rats, but these effects did not improve insulin content in these animals. Since there are no significant differences between Wistar rats consuming HFD + pitavastatin and Wistar rats consuming HFD in figure 8 of Mizukami et al. study, it seems that the increase in islets’ insulin content refers to the HFD in order to pitavastatin (20).
In one ex vivo study, simvastatin impaired insulin secretion from the islet of Langerhans reversibly and rapidly. The mechanism of this effect remained unknown because simvastatin had no effect on intracellular Ca2+ concentration and it was suggested that decrease insulin granule trafficking may produce this impairment action (23). However, it was revealed that statins reduced insulin secretion in HIT cells as a hamster β-cell line, but Ishikawa et al. showed supraphysiological concentrations of statins such as simvastatin and atorvastatin could inhibit islets insulin secretion, and this event recommended that treating doses of statins administered in hypercholesteremic patients have not deteriorated effects on insulin action (16).
5.1. Conclusions
In conclusion, in vivo studies assessment of pravastatin and rosuvastatin showed both drugs increase and decrease islets insulin secretion, and atorvastatin and fluvastatin reduced this variable. In vitro articles indicated that all statin drugs that investigated in the present study reduced this hormone secretion except pravastatin. Moreover, the mechanisms of increase in insulin secretion of pancreatic islets were oatps upregulation, increase blood flow of islets, and distribution in Ca2+ signaling. On the other hand the influenced mechanisms on decrease of insulin secretion were the adverse effect in Ca2+ signaling, induced oxidative stress in islet, reduced CoQ10 production, impairment of isoprenoid, inhibition of CDC and FXR functions, inhibition of FFA1, reduced GLUT-2 expressions, enhanced augmenting H2S signaling, decreased insulin granule trafficking, impaired β-cell function, and stimulated this cell apoptosis. Therefore, Pravastatin could enhance pancreatic islet insulin secretion while other statins reduced this variable. Finally, the contradictory effect of statins on islets’ insulin secretion can indicate the existence of laboratory and clinical research in this regard.