1. Background
Gastric cancer is a growth that is beyond the control of malignant cells in the gastric and is a type of cancer that grows slowly over the years; however, there are changes in the layers of the gastric prior to the development of the cancer (1). Gastric cancer is one of the most common cancers worldwide, especially in western countries, but its prevalence is increasing in some countries such as Iran, China, Ireland, and Chile (2-8).
The results of a recent study conducted at the Medical and Health Education Center of Iran have suggested that 39% of the causes of cancer deaths are associated with gastric cancer (8, 9).
The prevalence of this disease has been found to increase with the lower socio-economic situation, obesity, high-fat, consumption of high salt, diets enriched with nitrate, Helicobacter pylori infection, EBV virus, genetic factors (COX2, P53 involvement), gastric lesions, and tobacco as the risk factors (3, 10). The host-related risk factors and tumor characteristics, including primary tumor size, lymph node invasion, and distant metastasis, play a major role in its prognosis (5, 11). However, gastric cancer is one of the cancers that can be cured if it is detected and treated in the early stages (12).
There are several treatment methods available for dealing with patients afflicted with gastric cancer. Some methods are standard, while others are still tested in clinical trials. Six standard methods for dealing with gastric cancer are surgery, chemotherapy, X-rays, targeted therapy, immunotherapy, and radiation chemotherapy (13). The bacteriocin was observed on eukaryotic cells in 1976. Due to the lower side effects of bacteriocins, they can be used as an independent treatment or as an adjunct to other treatments (14).
There have been many reports about the positive effects of bacteriocins in treating infections. Clinical studies have also shown the role of bacteriocins associated with anti-cancer sequences in the prevention, control, and treatment of cancers – gastrointestinal cancers, in particular. Bacteriocins are cationic peptides that attach to the cell membrane by negative charge (15, 16). The third reason is that the membrane of tumoral cells has higher microvilli than normal cells and, therefore, their contact surface is higher. Furthermore, cancer cells are more sensitive than normal cells due to more proliferation and are affected by a lower concentration (17).
Anti-cancer peptides are designed with Anti CP software. These peptides are kind of novel bioactive peptides that could be used as anti-tumor drugs with several advantages, such as delivering the efficacy of a combined drug as well as having high specificity, high penetration capacity, and low toxicity for normal cells. The anti-cancer peptide sequence has been designed using AntiCP software, and carvacrol has been also synthesized chemically (6, 18).
Carvacrol is the most effective compound of the Satureja khuzestanica plant, and according to researchers, this compound can inhibit cancer cells (19, 20).
The innovation of the present study lies in the fact that it was the first to use the combination of carvacrol with a designed anti-cancer peptide in order to inhibit the Hela cancer cell line.
2. Objectives
This study aimed to determine the antibacterial and anti-cancer effects of modified carvacrol against AGS gastric cancer cell line by adopting flow cytometry technique.
3. Methods
3.1. The Sequence Design of Anti-cancer Peptide and Carvacrol Structure
The anti-cancer peptide sequence was designed using AntiCP software, and S. khuzestanica carvacrol was prepared by Barij Essence Company. Finally, the mixture of anti-cancer peptide and Carvacrol was prepared in a ratio of 1:1.
3.2. Bradford Protein Assay
The colorimetric method was employed to measure the concentration of protein in the solution. The basis of the Bradford experiment is the change in color absorption of the Coomassie Brilliant Blue G-250. The red color turns blue under acidic conditions due to binding to the test protein. If there is no protein to bind, a brown solution remains. Under the influence of van der Waals forces, this dye forms a strong and uncomplex combination with the carboxyl group of proteins and establishes an electrostatic interaction with the amine group of proteins. Protein binding stabilizes Coomassie blue form, and, therefore, the amount of blue complex in the solution is indicative of the protein concentration, which can be estimated by using the optical absorption properties versus the standard protein as a control. In this study, the Bradford assay was performed to determine the protein concentration at 595 nm (6).
3.3. Cell Culture
Treatment of cells was performed using different concentrations of anti-cancer peptides in order to evaluate AGS cell apoptosis against the combination of carvacrol and anti-cancer peptides by adopting flow cytometry (6).
3.4. Treatment of Cells by Using Different Protein Concentrations to Evaluate the Apoptosis Effect
To evaluate cell apoptosis, two 24-cell culture plates were prepared. After 24 hours, when the cells adhered to the bottom of the plates, they were removed with a pasteurizer pipette. Then, they were added to all three wells of the plates (triplicated) with concentrations of 2, 1, 0.5, 0.25, 0.125, and 0.0625 µg/mL of the freshly prepared mixture. The wells were also considered as controls; therefore, they were not treated with the combinations. Then, the final volume was prepared by one cc with the culture medium. The plates were incubated in a 37°C incubator in the presence of CO2 for 24 hours to treat the cells.
After removing the supernatant and washing it with phosphate-buffered saline (PBS), trypsin was added to remove cells from the bottom of the plates. An inverted microscope was employed to examine cell separation. Then the cells treated and untreated with the mixture were poured into a Falcon 15 mL tube with a concentration of 0.125 µg/mL and centrifuged at 1500 rpm for 5 minutes. The plate cell sediments were isolated for applying flow cytometry (6).
3.5. Evaluation of AGS Cell Apoptosis with Modified Carvacrol by Flow Cytometry
To implement the flow cytometry, the cell deposits from cultures of the cells treated with mixtures of combinations at a concentration of 0.125 µg/mL, as well as the untreated cells as controls (see Section 3-30), were transferred to separate falcons. Then 2 mL of culture medium was added. Falcon-containing cell suspension was sent to the tissue test laboratory in order to evaluate the extent of cell apoptosis by flow cytometry, and the results were analyzed (see the diagrams) (6). All tests were repeated twice.
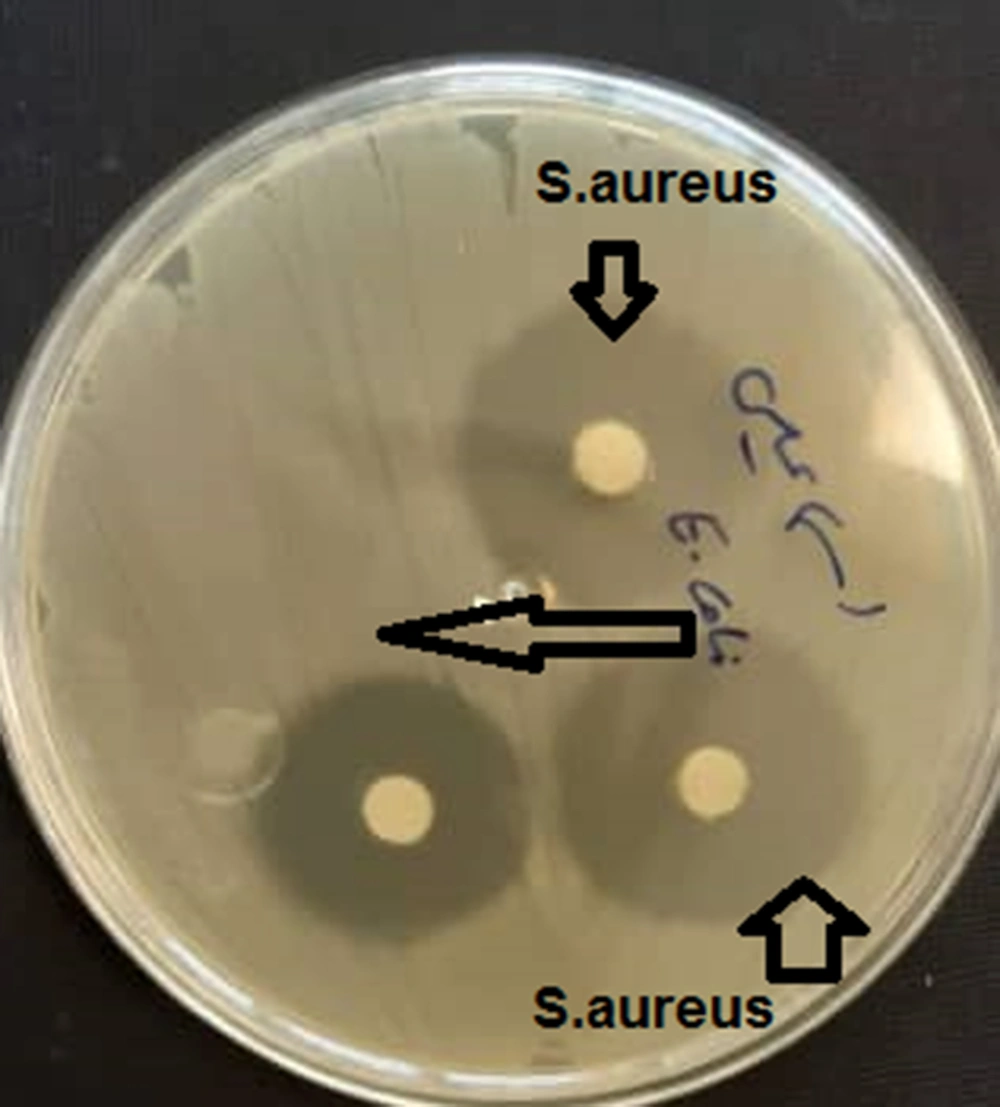
3.6. Evaluation of Antimicrobial Activity Carvacrol and Anti-cancer Peptide Using Disk Diffusion Technique
To check the antibacterial activity, the disc diffusion method was adopted, and the diameter of the inhibitory halo created by the fusion peptide was used.
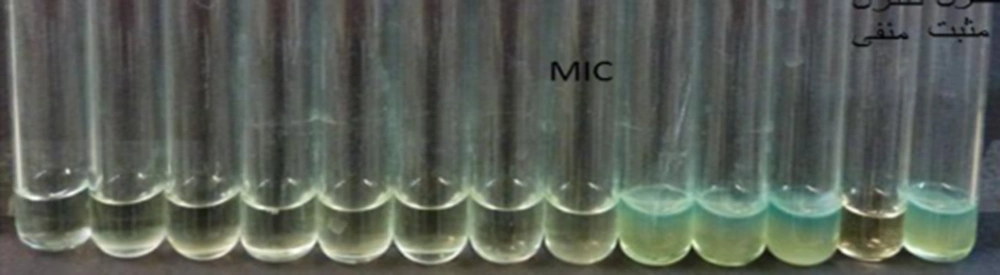
3.7. Investigating the Antibacterial Activity of Modified Carvacrol and Anti-cancer Peptide Using MIC Method
To obtain the minimum inhibitory concentration of the compound against the mentioned bacteria, the MIC method was implemented according to CLSI guidelines.
3.8. Statistical Analysis
Statistical analysis was performed using Excel and SPSS 2021 software.
4. Results
4.1. Results of Carvacrol and Anti-cancer Peptide
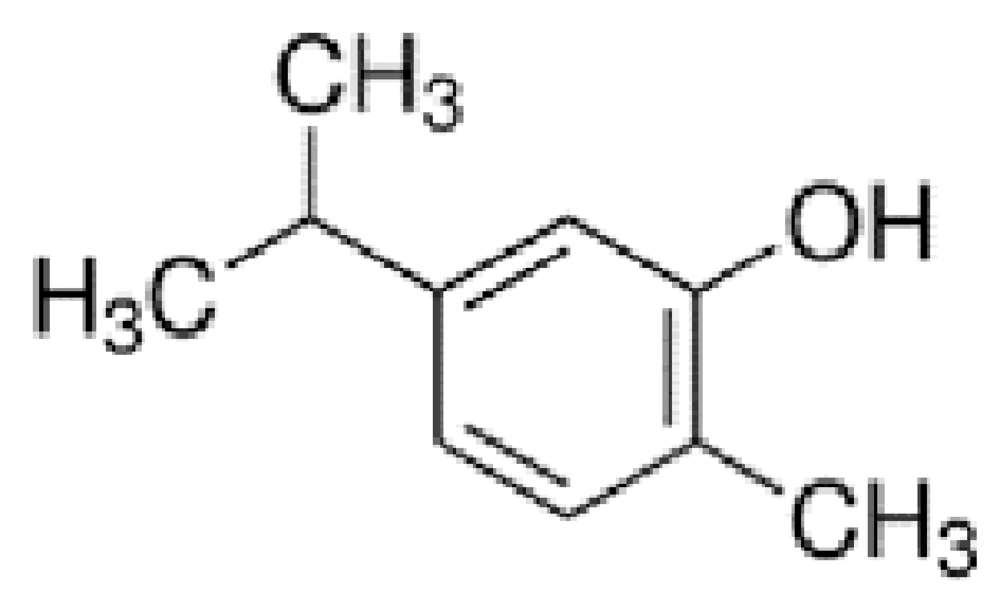
The sequence of anti-cancer peptides designed by anti-CP software is Lys- Ile-Val-Lys-Cys-Leu-ser-pro-Ala-Ile and Carvacrol structure is C10H14O.
The Carvacrol structure is displayed in Figure 1.
4.2. Result of Bradford Assay
The concentration of anti-cancer peptide synthesized by the Bradford method was found to be 10 µg/mL.
4.3. Results of Apoptosis by Flow Cytometry
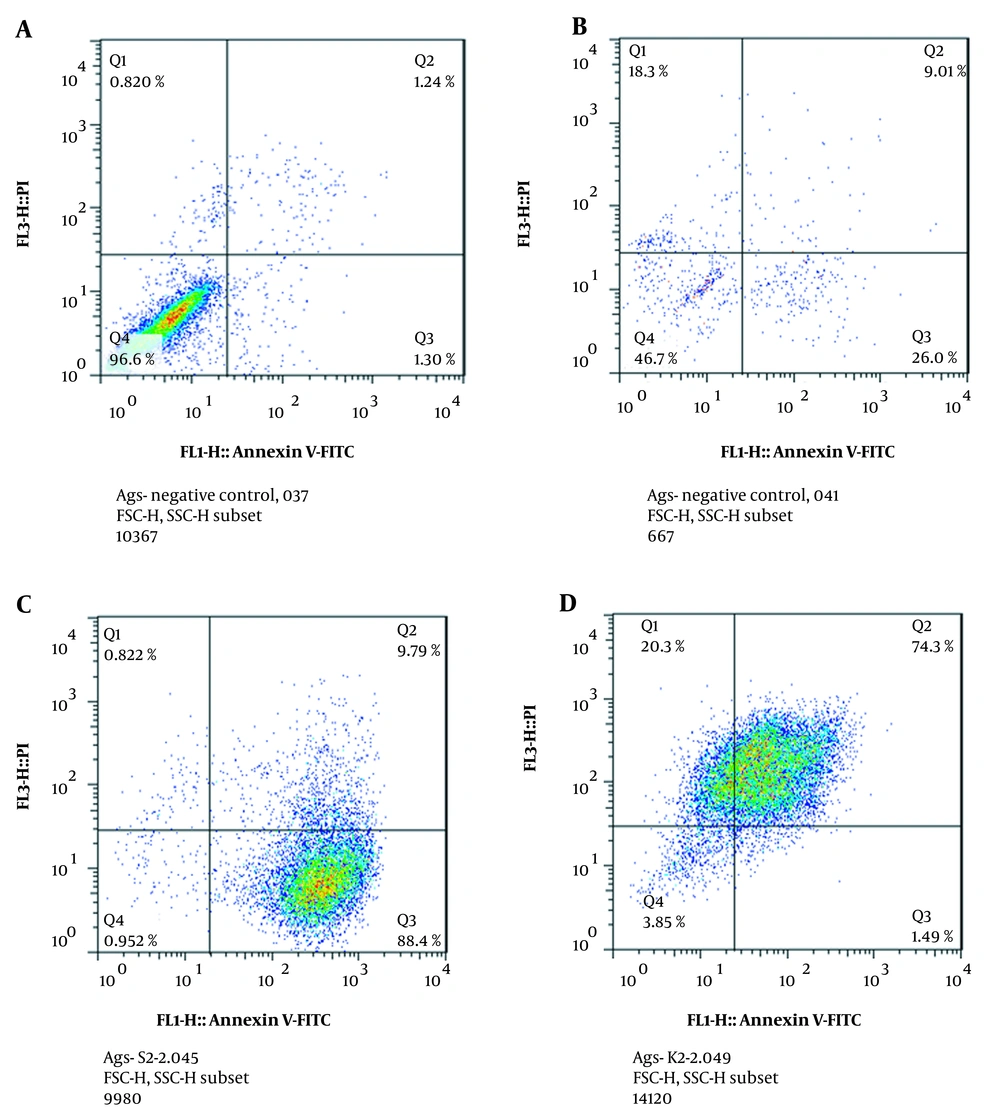
The results of flow cytometry are presented in a four-cell table in Figure 2. Annexin region V-/PI-: living cells are located in this region. Anxin V +/PI- region: primary apoptotic cells are located in this region. 3- Anxin V +/PI + region: dead cells are located in this region by secondary necrosis and apoptosis. Anxin V-/PI + region: damaged cells in this region are present during the preparation process (it was assumed that necrosis and no apoptosis had occurred in them).
According to our study results, the percentage of cells treated with mixtures of combinations with primary apoptosis (Q3 region), necrosis (Q1), and secondary apoptosis (Q2 region) increased compared to that of untreated cells. These results showed that the treatment of gastric cancer cells with mixtures of combinations at a concentration of 0.125 µg/mL induced apoptosis in the AGS cell line (Figures 2, 3).
Treatment of AGS cell with Carvacrol Phosphate buffered saline, Negative control, and anti-cancer peptide plus carvacrol. Q4 is a living cell and Q2, Q3, and Q1 are cell death (Apoptosis plus necrosis). A, Negative control. Our study results also demonstrated that 96% of the cell line was a living cell line. B, Positive control Phosphate buffered saline was used for a treatment that induced a 35% apoptotic effect on the AGS cell line. C, Treatment with a mixture of anti-cancer peptide and carvacrol induced 64% apoptotic activity compared to PBS. D, Treatment with carvacrol induced 40% apoptotic activity compared to PBS.
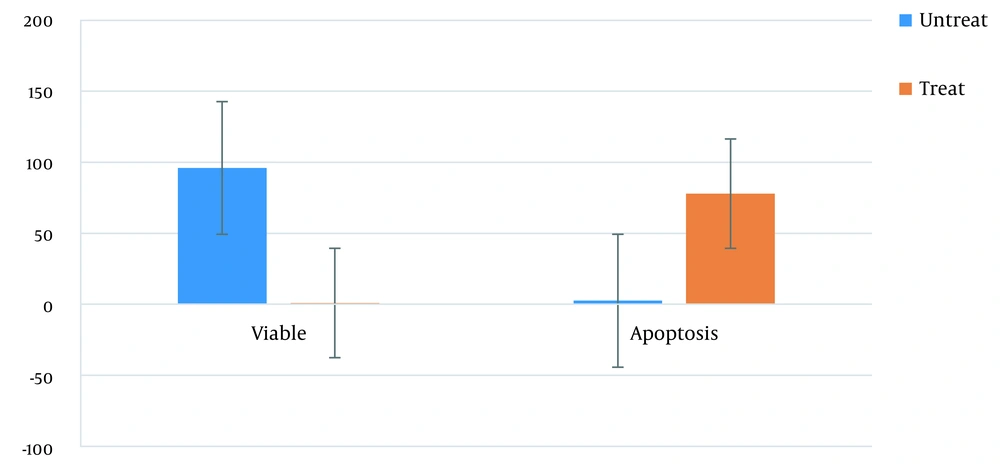
The rate of cell apoptosis was 2.5% before the treatment, while the rate was 78% after the treatment (i.e., 31-fold increase). The cell viability was 96% before the treatment, while it was almost 1% after the treatment (96-fold decrease) (P-value < 0.05).
4.4. Disc Diffusion and MIC Test Results Against Different Bacteria
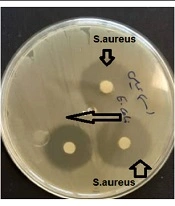
The results showed that the minimum inhibitory concentration of the compound was 6 µg/mL for Staphylococcus aureus, while this value was 12 µg/mL for Escherichia coli. The results of disk diffusion were 15 and 10 mm for S. aureus and E. coli, respectively (Figures 4, 5).
5. Discussion
The treatment of cancer requires sustained efforts to develop effective drugs. To this end, the role of herbal compounds and anti-cancer peptides have been widely studied.
Apoptosis is a process that removes harmful, damaged, and old cells. Disorder in this pathway causes disease that can sometimes lead to the reproduction of abnormal cells. The apoptosis process activates caspases, especially caspase 3, in two pathways, internally and externally (6, 7, 21-23).
In the present study, treatment of gastric cancer cells (AGS) with 0.125 µg/mL of a mixture of combinations induced apoptosis in the studied cell line. So, it can be stated that this combination induced apoptosis and led to apoptotic death.
The results of flow cytometry also showed that cancer cells treated with modified carvacrol and anti-cancer peptide induced apoptosis significantly higher than untreated cells. Treatment of gastric cancer cells with the following combination at a concentration of 0.125 µg/mL induces apoptosis in this cell line (6, 13, 14, 24).
Carvacrol is known as an important phenolic compound in the S. khuzestanica plant. It has been reported to have powerful antioxidant, anti-tumor, and anti-inflammatory properties. The neuroprotective, hepatic, and renal protective effects of these polyphenolic substances on different disease models have been also documented (25).
Phosphate buffered saline has been detected to exert an apoptotic effect due to the produced reactive oxygen and nitrogen species (RONS). Other studies have also suggested that natural microbial compounds have anti-cancer properties and are able to regulate gene expression (6, 18, 26).
In the study by Davoodi et al., the metabolite profiling of methanolic extract from aerial parts of S. khuzistanica Jamzad was evaluated using HPLC-PDAESI (19). In the study of Meskini et al., the antibacterial and wound healing effects of S. khuzestanica in the form of an ointment formulation were investigated, and its antibacterial activity and acceleration in wound healing were also confirmed, which was consistent with our study result (20, 25). In the study of Jalalvand et al., the antibacterial effects and apoptosis of gastric cancer cells were examined using flow cytometry – a technique which was also used in our study (27).
An innovative therapeutic strategy against cancer cells includes using anti-cancer peptides. Anti-cancer peptides can be selected or designed by using anti-CP software. The physicochemical properties and amino acid composition influence their conformation, net charge, and secondary structure, that lead to targeting specificity and cell interaction. Using anti-cancer peptides is a new and superior choice compared to other treatment methods in therapeutics because of their high penetration and easy modifications. Ideally, anti-cancer therapy destroys a range of cancer types, but not healthy cells.
5.1. Conclusions
In this study, the effect of modified carvacrol and anti-cancer peptide against Caspase3 was investigated, and the apoptotic induction of this mixture of combinations was evaluated. It was concluded that the treatment with a mixture of combinations had cytotoxic effects against AGS cells. Our study results also suggested that the mixture of combinations may have activated the apoptosis by the caspase 3internal pathway, leading to the death of cancer cells.
The modified Carvacrol can be applied as an effective drug to control the growth of gastric cancer cell line (AGS) and may be used as complement in chemotherapy drugs. This compound may change gene expression in apoptosis and, ultimately, limit the growth of humoral cells.
Our study results may have provided the basis for conducting more extensive studies in order to comprehensively study the effects of the given compound on cancer. In vitro results showed that this compound had major properties which should be examined by in vivo studies.
5.2. Disclaimer
All authors are responsible for the data and the contents of the paper.