1. Background
Colorectal cancer (CRC) is one of the most common types of cancer worldwide. CRC incidence is predominantly associated with smoking, unhealthy eating habits, obesity, and aging. As a result of metastasis and drug resistance, this cancer has a high mortality rate despite conventional chemotherapy. The discovery of new anti-cancer drugs and the underlying molecular mechanisms can therefore help to treat this cancer (1). Recent cancer research has focused on natural compounds that can kill cancer cells with negligible side effects (2).
Myricetin (MCN) is a dietary flavonoid found in walnuts, fruits, vegetables, tea, and berries (3, 4). Recent studies suggest that MCN has anti-inflammatory, antiplatelet, antimicrobial, antioxidant, and antiviral properties. In addition, MCN exhibits cytotoxic effects on various cancer cells, including prostate, breast, skin, and gastric cancers (5, 6).
Necroptosis is a type of cell death program with similar features to apoptosis and necrosis, which has a critical role in managing many kinds of cancers and other disorders. Necroptosis activates as a signaling pathway to kill cancer cells when they are resistant to apoptosis. Several anti-cancer agents activate critical regulators of necroptosis signaling, including receptor-interacting protein kinase-1 (RIPK1), receptor-interacting protein kinase-3 (RIPK3), and mixed lineage kinase domain-like protein (MLKL). The activated RIPK1 binds to RIPK3 to phosphorylate RIPK3 and produce the necrosome complex. The necrosome complex that interacts with MLKL and oligomerizes can disrupt membrane permeabilization, resulting in cell death (7).
2. Objectives
The current research aimed to investigate the involvement of the necroptosis pathway in the anti-cancer impact of the MCN on colon cancer (HT-29) cells (HT-29).
3. Methods
3.1. Cell Culture
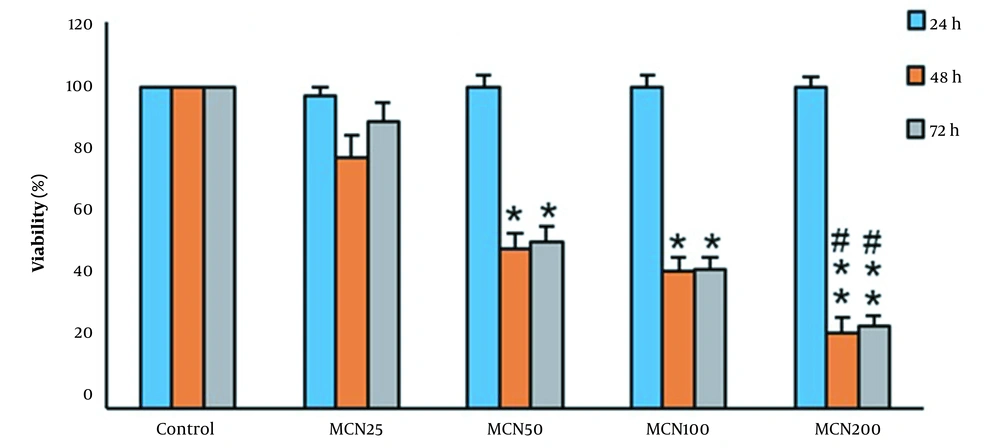
HT-29 cell line was from the Iranian National and Genetic Center. The cells had grown in complete media and were maintained in a moist temperature incubator with 5% CO2 and 37°C. After dissolving MCN in Dimethyl sulfoxide (DMSO), DMEM had used to dilute and obtain the desired concentration. A control group was exposed to only media, and the experimental groups were treated with three mM Necrostatine-1 (Nec-1, a necroptosis inhibitor), 50 μM MCN, and Nec-1 + MCN for 48 hours, respectively. Based on the MTT assay, the incubation time and concentration of MCN were selected (Figure 1). Nec-1 was also dissolved in 1% DMSO.
The viability percentage of HT-29 cells in different concentrations and incubation times. Each assay has been done six times (4 replicates per assay), and the mean ± standard deviations have been indicated. * P < 0.05, ** P < 0.01, # P < 0.05; *, # and $ symbols indicate comparison to the control, and MCN50 groups.
3.2. Cell Viability Assay
MTT (dimethylthazolk 5-diphenyl tetrazolium bromide) test is applied to measure cellular metabolic activity for determining cell survival. It is a colorimetric method based on the cleavage of the tetrazolium ring of MTT by mitochondrial succinate dehydrogenase in living cells. Cells were exposed to MCN (0, 25, 50, 100, and 200 m) for 24, 48, and 72 hours. The cells were then incubated with MTT solution (0.5 mg/mL) for 3 hours, and the supernatants were replaced with DMSO (100 L). Finally, the absorbance was measured at 570 nm.
3.3. Annexin V / PI Apoptosis Assay
The cells were harvested in 6-well dishes (106 cells/ well) and exposed to MCN or Nec-1 for 48 hours. Then, an Annexin V (FITC)/ PI (propidium iodide) kit (Invitrogen, USA) was used to determine the percentages of the dead or survival cells by flow cytometry. The FITC-/PI- cells were viable, while FITC+/PI-, FITC+/PI+, and FITC-/PI+ cells indicated early apoptosis, late apoptosis, and necrosis stages, respectively.
3.4. Real-Time PCR
An RNeasy kit was used to isolate the RNA of the cells (107 cells), and a cDNA kit was used to convert the RNA to cDNA. Two μL of the prepared cDNA amplified in a 25 μL PCR reaction contained SYBR Green and primers (Appendix 1 in Supplementary File). The following 45-cycle program was used to amplify the PCR: 95°C for 10 seconds, 95°C for 15 seconds, 60°C for 20 seconds, and 60°C for 20 seconds. The 2-ΔΔCT method was applied to analyze the data.
3.5. Western Blotting Analysis
After treatment, HT-29 cells were washed and added to radioimmunoprecipitation (RIPA) lysis buffer. A BCA assay kit was used to determine the protein concentration within the cells. Lysate protein separated on 10% SDS-PAGE and transferred onto a PVDF membrane. The HT-29 cells were incubated with diluted primary antibodies (anti-RIPK1: ab178420; anti-RIPK3: ab222320; anti-MLKL: ab184718; anti-beta-Actin: ab115777) for 3 hours. The cells were then treated with horseradish peroxidase (HRP)-conjugated secondary antibody (ab205719) for 90 min. Proteins were detected using an ECL detection kit. Image J software was used to quantify the band density.
3.6. Statistical Analysis
3. Data were analyzed in SPSS (version 21.0) by one-way analysis of variance, followed by posthoc pairwise comparison with LSD or Kruskal-Wallis for non-parametric data. The P-values less than 0.05 were statistically significant.
4. Result
4.1. Cell Viability
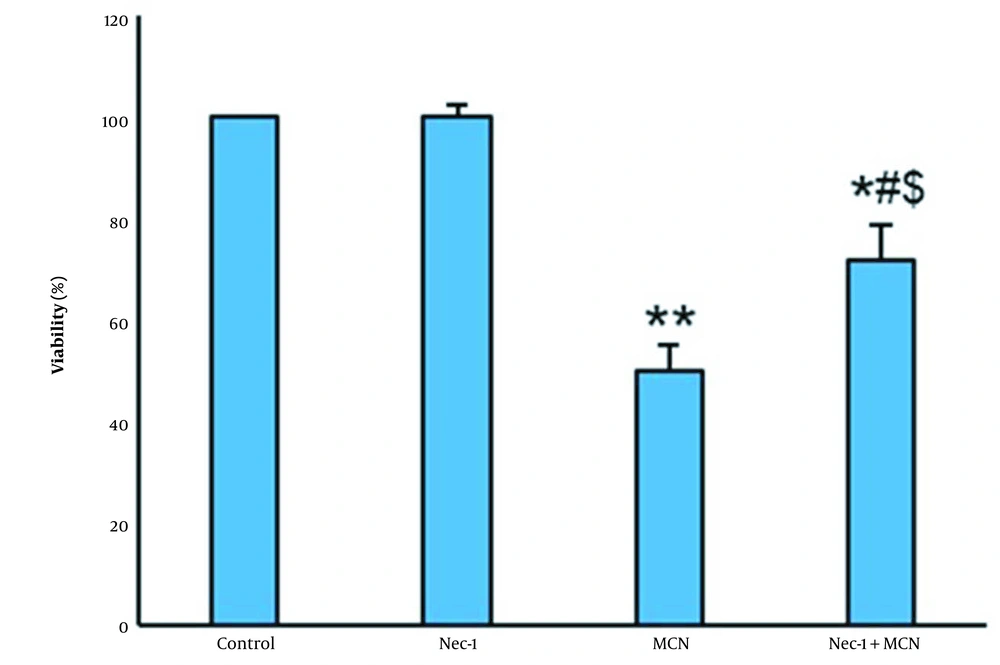
As illustrated in Figure 1, after 24 hours of incubation with various doses of MCN, no considerable decline in the viability of the HT-29 cells was observed. A significant decrease in the cell survival rate from 100% in control to 78.1%, 49.8%, and 42.8% in 25, 50, and 100 μM-treated cells were observed during 48 hours, respectively. No cell survival rate alterations occurred during 72 hours of incubation with 100 and 200 μM/mL MCN compared to the 48 hours. Cell viabilities at the concentration of 200 μM MCN during 48 and 72 hours were 23.7% and 25.8%, respectively, and hence were considered lethal concentrations (Figure 1). The half-maximal inhibitory concentration (IC50) results had used to determine the half-maximal concentration of MCN required to inhibit the growth of HT-29 cells within 48 hours. Treatment with MCN + Nec-1 significantly increased the survival of the HT-29 cells (Figure 2) compared to the only MCN-treated cells (P < 0.05).
4.2. Annexin V-FITC/ PI Assay
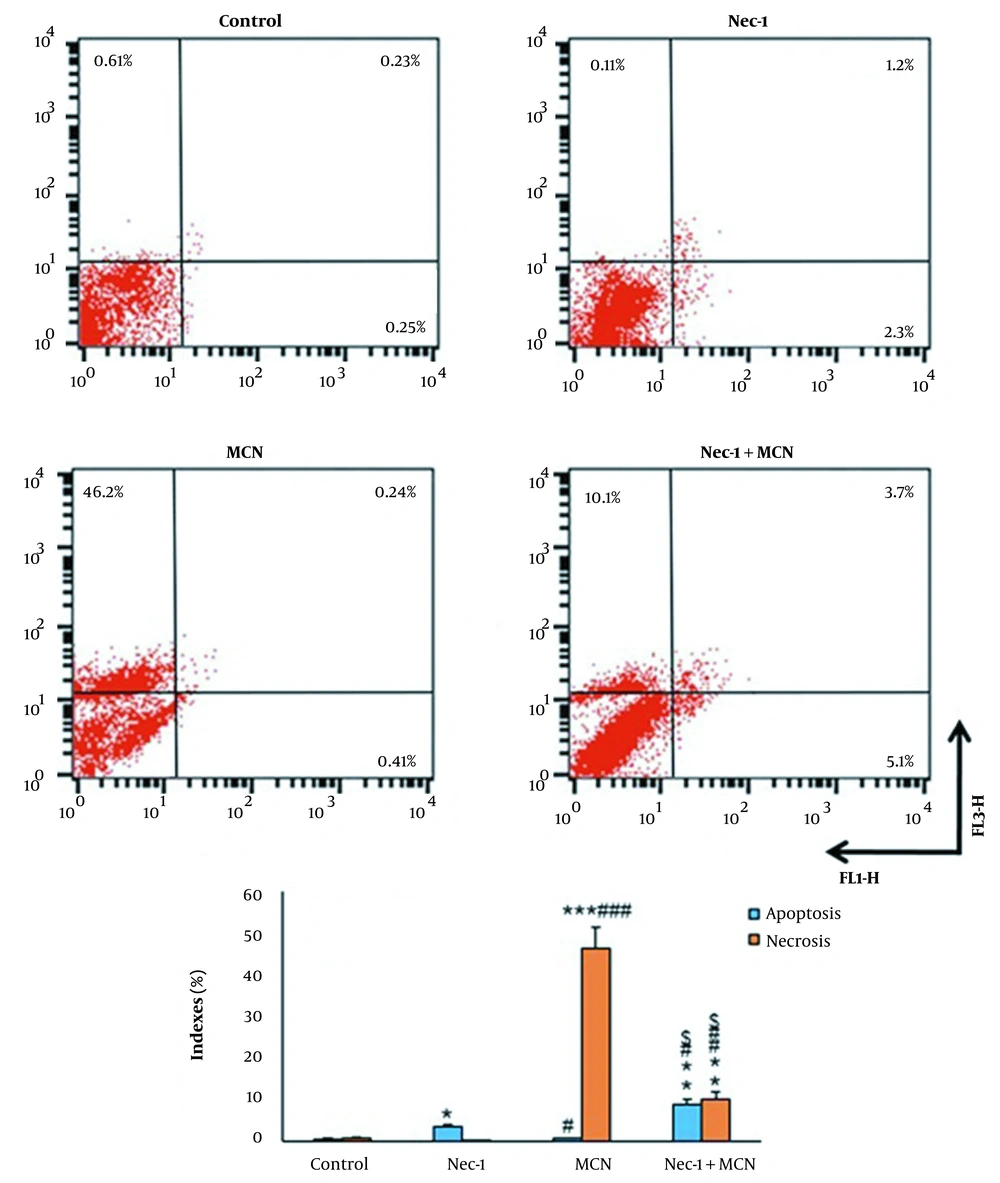
An annexin V/PI staining was performed to determine whether the MCN induced apoptosis or necrosis in HT-29 cells. In the untreated group, 98.9% of the cells were viable, 0.48% were in apoptosis, and 0.61% were in the necrosis stage. In comparison to the control, the proportion of viable cells decreased from 98.9 to 96.39%, 53.15%, and 81.1% in the Nec-1, MCN, and Nec-1 + MCN-treated cells, respectively. MCN slightly enhanced the percentage of apoptosis and the percentage of necrosis in HT-29 cells (75.7-fold, P < 0.001). Nec-1 + MCN caused a significant elevation in apoptosis (13.5-fold, P < 0.001), and a significant reduction in the necrosis (4.6-fold, P < 0.01) of the HT-29 cells compared to the MCN group (Figure 3).
Flow cytometry results of Annexin V/PI staining (mean ± SD, n = 3). Lower left quadrant: Live cells, lower right quadrant: Early apoptosis, upper right quadrant: Late apoptosis, upper left quadrant: Necrotic cells. * P < 0.05, ** P < 0.01, *** P < 0.001, # P < 0.05, ## P < 0.01, ### P < 0.001, $ P < 0.01.
4.3. Real-Time PCR Assay
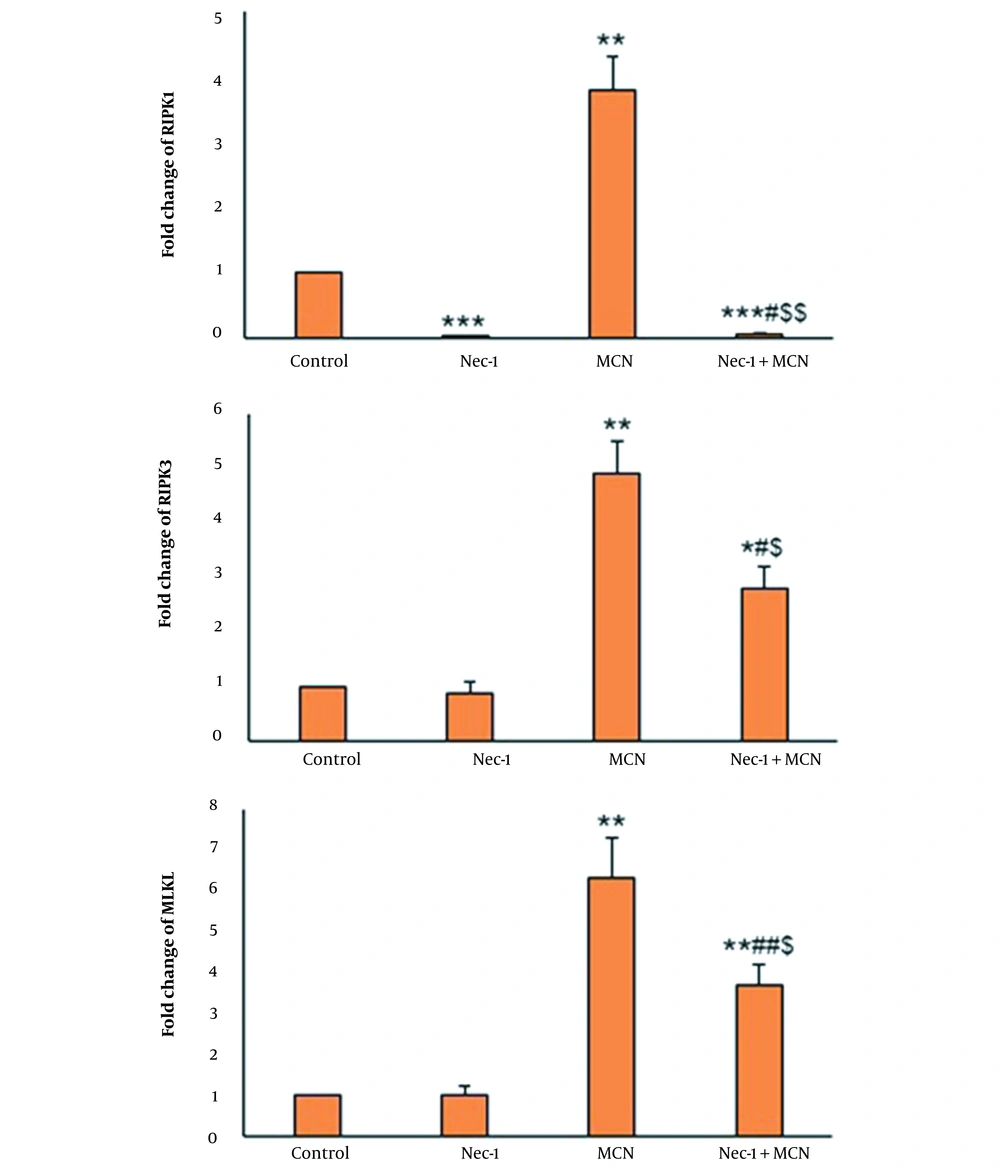
The expression of the RIPK1 gene was low in the Nec-1-exposed HT-29 cells. RIPK3 and MLKL expression in cells exposed to Nec-1 were unchanged compared to the control. MCN significantly enhanced the expression of MLKL, RIPK1, and RIPK3 compared to the control (P < 0.01). In the Nec-1 + MCN group, the mRNA expression of MLKL and RIPK3 genes was considerably lower than in the MCN-treated cells (Figure 4).
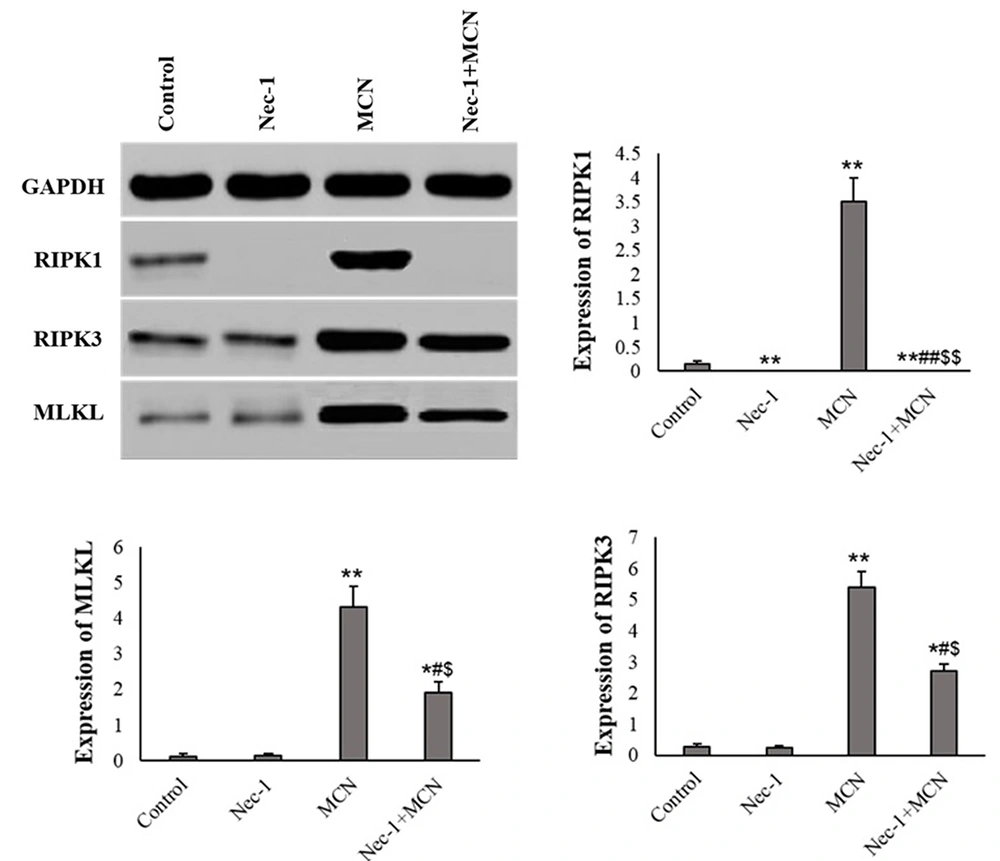
4.4. Western Blotting Analysis
In HT-29 cells treated with Nec-1, the RIPK1 protein was not expressed. The protein levels of MLKL and RIPK3 in the Nec-1-exposed HT-29 cells were not changed compared to the control.
MCN caused a significant elevation in the protein level of RIPK1, RIPK3, and MLKL compared to the control (P < 0.001). In the Nec-1 + MCN group, the protein expression of RIPK3 and MLKL was obviously lower than in the MCN-treated cells (P < 0.01) (Figure 5).
5. Discussion
This study demonstrated the cytotoxic effects of MCN on HT-29 cells. In line with our results, MCN inhibited the proliferation and survival rate of human papillary thyroid cancer (SNU-790 cells), cervical cancer (HeLa cells), and bladder cancer (8-10). In Xu et al. study, MCN had a high anti-proliferative effect on prostate cancer cells (11).
Based on our flow cytometry results, MCN did not alter the apoptosis percentage of HT-29 cells. In contrast to our findings, apoptosis was induced in human colon cancer (HCT-15), hepatocellular carcinoma (HepG2), and prostate cancer (PC3) cell lines after MCN treatment (11-13). The converse results may be the result of variations in cancer cell types or MCN concentrations used in those studies.
The Annexin V/PI method revealed that MCN significantly enhanced necrosis in HT-29 cells. Most chemotherapy drugs and radiation can induce necrosis in cancer cells (14, 15). When apoptosis is suppressed, another type of programmed cell death (necroptosis) has activated to kill the malignant cells (16, 17). Therefore, the mRNA expression and protein level of the main modulators of necroptosis in the HT-29 cells was investigated in the current study. MLKL, RIPK1, and RIPK3 mRNA and protein levels were increased in HT-29 cells treated with MCN. The protein expression of RIPK1 and RIPK3 is suppressed in CRC tissues (18). The suppression of RIPK3 protein expression in breast cancer patients associates with cancer progression (19). The expression of the MLKL can be suppressed in malignant cells (20-22).
Anthracyclines and oxaliplatin stimulate the necroptosis pathway by inducing the expression of RIPK3 and MLKL proteins in the malignant cells (23).
The increasing expression of necroptotic-related proteins was accompanied by decreasing survival of the MCN-treated cells. These findings suggest that MCN can inhibit the growth of the HT-29 cells by activating the necroptosis signaling pathway.
This study examined the effects of Nec-1 on the HT-29 cells to evaluate the role of necroptosis. Cells exposed to Nec-1 exhibited enhanced growth, as well as reduced expression of RIK1, RIPK3, and MLKL. The enhancing growth of the HT-29 cells by Nec-1 indicates that necroptosis plays a crucial role in the anti-cancer effects of MCN. The results of this study showed that Nec-1 induces considerable apoptosis in the presence of MCN. Nec-1 can activate the caspase cascade (24) and may enhance apoptosis in MCN-treated HT-29 cells. Nec-1 induced apoptosis in Shikonin-exposed Leukemia cells (25). Interestingly, the increased apoptosis was accompanied by an increased survival rate in Nec-1+MCN-exposed HT-29 cells. As shown in the results, Nec-1 could decrease oxidative stress in the MCN-exposed cells and hence may increase the growth of the HT-29 cells.
5.1. Conclusions
Overall, this study demonstrated that MCN inhibits colon cancer cell growth effectively. MCN causes high cytotoxicity in HT-29 cells due to the stimulation of necroptosis signaling.