1. Background
The bone is an active metabolic tissue that continuously changes its structure through the two processes of modeling and remodeling. Recent findings indicate that bone tissue is an important source of growth factors. Insulin-like growth factor-I and II (IGF-I and IGF-II) are the most common growth factors produced in the bone tissue and contribute to anabolic responses to mechanical loads in bone, triggering osteogenesis (1). Insulin-like growth factors bind to IGF-binding proteins (IGFBPs), which control the bioavailability of IGFs (1). The binding tendency of IGF-1 is higher than that of IGFBP-3 (2). The gene expression and plasma concentration of IGF-I, IGF-II, and IGFBPs play autocrine and paracrine roles in regulating the growth and development of osteoblasts during bone differentiation (3).
The gene expression of IGF-I is regulated temporarily depending on the type of tissue. Moreover, nutritional factors and physical activity influence IGF-I gene expression (1). An in vitro study showed that vitamin D stimulated IGF-I and IGFBP-3 gene expressions in the bone (4, 5). Mechanical stimulation is another factor that affects IGF-1 production by local bones. Studies demonstrate that the mechanical stimulation of the bone elevates IGF-I mRNA levels, inducing bone formation (6, 7). However, it is uncertain if mechanical stimulation induces changes in IGFBP-3 expression (8). Also, the biogenesis of the bone tissue is influenced by other factors, such as the overproduction of reactive oxygen species (ROS) induced by aging or diseases like cancer or other chronic diseases. Hydrogen peroxide (H2O2) is a type of ROS that potentially inflicts harm to the bone (9-11) and is associated with the increased formation and function of osteoclasts (12). Oxidative stress may contribute to bone loss under normal physiological or pathological conditions by reducing the production of bone growth factors. Therefore, we aimed to investigate the effects of factors such as aerobic training and vitamin D supplementation on the expression of bone growth factors under oxidative stress in vivo.
2. Objectives
Research findings support the independent effects of mechanical loading and vitamin D on the upregulation of bone growth factors under normal physiological conditions in vitro. Also, it was shown that the interaction of aerobic training and vitamin D influenced the gene expression and protein concentration of bone markers in rats poisoned with hydrogen peroxide in vivo (9-11). Considering these observations, we hypothesized that the interaction of aerobic training and vitamin D supplementation could affect the gene expression levels of bone growth factors, IGF-I and IGFBP-3, in male Wistar rats poisoned with H2O2.
3. Methods
3.1. Animals
Thirty-six eight-week-old male Wistar rats weighing 200 ± 20 g were purchased from the Animal Breeding Center of Shiraz University of Medical Sciences and transferred to the Physiology Research Center of Kerman University of Medical Sciences. The animals were housed in the ambient temperature, humidity, and light/dark cycle of 22 ± 2°C, 50 ± 5%, and 12 hours, respectively. The rats were kept in animal cages with ad libitum access to water and rodent food purchased from Pars Livestock Company (Tehran, Iran). All the principles of working with animals were observed according to the 1985 NIH publication. This study was approved by the Ethics Committee of the Central Tehran Branch of Islamic Azad University under the code of IR.KMU.REC.1396.1562.
3.2. Study Groups
Following one week of adaptation, the rats were randomly divided into six groups (n = 6): (1) healthy animals (control), (2) sham (treated with dimethyl sulfide oxide (DMSO)), (3) treated with hydrogen peroxide (H2O2), (4) treated with H2O2 + aerobic training (H2O2+ E), (5) treated with H2O2 + cholecalciferol (H2O2+ Vit D), and (6) treated with H2O2 + aerobic training + cholecalciferol (H2O2+ E + Vit D).
3.3. Hydrogen Peroxide Injection
Rats received intraperitoneal H2O2 (Merck Co.) at a dose of 1 mmol/kg three times a week on even days (13).
3.4. Cholecalciferol Supplementation
The rats received 0.5 μg/kg of cholecalciferol intraperitoneally (vitamin D3, 300,000 UI/mL, the brand name of Dithrecol, Caspian Tamin Tehran Co., Iran) every day. To achieve the appropriate injection dose, normal saline and DMSO were employed to dissolve cholecalciferol. To evaluate the effects of this solvent, the rats in the sham group received DMSO intraperitoneally at the same dose every day (10).
3.5. Exercise Training
After a week of training on a rodent treadmill, the rats started eight weeks of aerobic training. During the training period, the slope was 10° uphill. The total training time in the first two weeks was 30 minutes, to which 15 minutes were added every two weeks until the end of the fifth week. From the fifth week to the end of the eighth week, the duration of training remained constant at 60 minutes. Also, the speed in the first week was eight meters per minute, which was increased by four meters per minute every week until the end of the fourth week. Afterward, the intensity remained constant at 20 meters per minute until the end of the training period (10).
3.6. Dissection and Sampling
Twenty-four hours after the last training session and following 12 hours of fasting, the rats were anesthetized by inhaling chloroform and then sacrificed. Next, a skilled veterinary surgeon recovered tibiae, which were kept at -70°C for further examination.
In order to extract RNA from tissue samples, the kit of the Yekta Tajhiz Co. (Iran) was used following all steps according to the provider’s protocols. After RNA extraction with high purity and concentration, cDNA synthesis was done using a specific kit (Fermentas, USA) according to the manufacturer’s protocol. In this stage, the cDNA synthesized was used for the reverse transcription reaction. Three primer pairs (forward and reverse) were designed for the IGF-I, IGFBP-3, and GAPDH (as internal control) genes using the sequences available in the NCBI database (Table 1). The real-time (RT)-PCR technique was employed to quantify the expression of these genes. Each PCR reaction was performed using a PCR master mix (Applied Biosystems) and SYBER Green in ABI Step-One (Applied Biosystems, Sequences Detection Systems, Factor City, CA) according to the manufacturer’s protocol. For each RT-PCR round, 40 cycles were considered with temperatures of 94°C for 20 seconds, 60 - 58°C for 30 seconds, and 72°C for 30 seconds. Post-PCR melting curves confirmed the specificity of single-target amplification reactions. Finally, we determined the fold expressions of IGF-I and IGFBP-3 relative to GAPDH.
| Gene and Primer Sequence (5’ → 3’) | Length | Amplicon (bp) | TM (°C) |
|---|---|---|---|
| IGF-I | 122 | ||
| Fwd: GTTGATAGGTGGTTGATGAATGG | 23 | 57.49 | |
| Rev: AGAATGTAAAGAAAGGGCAGGG | 22 | 58.30 | |
| IGFBP-3 | 167 | ||
| Fwd: ACAATGCTGGGGGTGTGGAAAG | 22 | 63.33 | |
| Rev: GTTGTGGGTGTCTGTGCTCTGG | 22 | 63.57 | |
| GAPDH | 133 | ||
| Fwd: GACAACTTTGGCATCGTGGA | 20 | 58.77 | |
| Rev: ATGCAGGGATGATGTTCTGG | 20 | 57.64 |
3.7. Statistical Analysis
The Shapiro-Wilk test was used to assess the distribution of the data. For comparing the control group with H2O2- and DMSO-treated groups, the independent samples t-test was utilized. Also, two-way-ANOVA (Exercise × Vitamin D) with Bonferroni’s post-hoc test was used to assess the interaction between these variables. Statistical analysis was conducted in SPSS software version 26 at a significance level of P ≤ 0.05.
4. Results
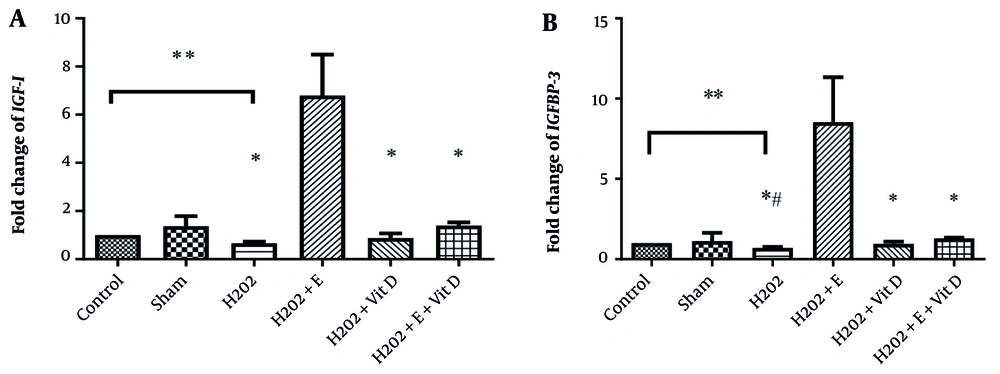
The expression levels of IGF-I and IGFBP-3 in the bone tissue of rats can be seen in Figure 1. The results of the independent samples t-test showed that treatment with H2O2 significantly reduced IGF-I (P = 0.001, t = -18.3) and IGFBP-3 (P = 0.001, t = -18.3) expressions compared to the control group. Treatment with DMSO; however, did not significantly affect the gene expressions of IGF-I (t = 0.78, P = 0.45) and IGFBP-3 (t = -0.07, P = 0.94) compared to the control group (Table 2).
Effects of 8-week aerobic training and cholecalciferol supplementation on the gene expression of A, IGF-I; and B, IGFBP-3 in the bone tissue of rats (n = 6 per group). Values are expressed as mean ± SD. *: Significant difference compared to the H2O2 + E group; **: Significant difference between the control and H2O2-treated groups; P < 0.05, significant difference comparing the H2O2+ E+ Vit D group with the H2O2 group; P < 0.1.
| Parameters | Between-Group Comparison (Sham-Control) | Between-Group Comparison (H2O2-Control) | Two Way-ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exercise | Vitamin D | Exercise × Vitamin D | ||||||||
| T | P | T | P | F | P | F | P | F | P | |
| IGF-I | 0.78 | 0.45 | -2.34 | 0.04 | 28.71 | 0.0001 | 28.71 | 0.029 | 8.59 | 0.008 |
| IGFBP-3 | -0.07 | 0.94 | -2.36 | 0.04 | 71.51 | 0.0001 | 22.01 | 0.0001 | 40.59 | 0.0001 |
The results of two-way ANOVA showed that exercise (F = 28.71, P = 0.0001, η coefficient = 0.59) or vitamin D supplementation (F = 5.52, P = 0.029, η coefficient = 0.22) increased IGF-I expression. Also, concomitant exercise and vitamin D considerably increased the expression of IGF-I (F = 8.59, P = 0.008, η coefficient = 0.30) (Table 2). In addition, either exercise (F = 71.51, P = 0.0001, η coefficient = 0.78) or vitamin D supplementation (F = 22.01, P = 0.0001, η coefficient = 0.52), as well as their combination (F = 40.59, P = 0.0001, η coefficient = 0.67) had a significant amplifying impact on IGFBP-3 gene expression (Table 2).
Regarding the significant interaction between exercise and vitamin D supplementation, further analysis revealed that the effect of aerobic training on the expression levels of IGF-I and IGFBP-3 was significantly more pronounced compared to other interventions (P < 0.05) (Figure 1).
5. Discussion
The levels of IGF-I and IGFBP-3 in the H2O2 group decreased considerably. Nevertheless, treatment with H2O2 + exercise increased the expression of IGF-I and IGFBP-3. Moreover, the simultaneous administration of H2O2, exercise, and vitamin D markedly influenced the gene expression of IGFBP-3. In line with the findings of this study, Zeng et al. showed that H2O2 injection disrupted the function of IGF-I (14). This phenomenon appears to be related to the inhibition of the phosphorylation of the IGF-I tyrosine kinase receptor or the restricted bioavailability of IGF-I (14, 15).
We observed that the expression of IGF-I and IGFBP-3 in the tibiae of rats elevated following exercise and vitamin D supplementation. The bone matrix was introduced previously as a reservoir of growth factors, and it has been shown that the concentration of IGF-I in the bone extract was even higher than in the liver tissue. Also, growth factors have been reported to increase osteogenesis by inducing the differentiation of osteoblasts and stimulating the proliferation of osteoblast precursors (16).
The exercise was observed to increase the expression of IGF-I and IGFBP-3 in the tibiae of rats. In rats undergoing nephrectomies, Troib et al. reported an increase in tibia length and the expression of IGF-I following four weeks of running on a treadmill (17), which was attributed to the mechanical load exerted by exercise. In a study by Yeh et al., exercise augmented the rate of tibia bone formation, which correlated with the concentration of IGF-I in the tibia (18). This phenomenon could be partly explained in either of the following two ways: (1) Exercise increases the number of local cells producing IGF-I in the bone, and (2) exercise induces the local production and accumulation of IGF-I (18). Reijnders et al., in an in vivo study, showed that IGF-I mRNA level increased in endocortical osteocytes and lamellar bone six hours after exerting mechanical loading on the tibia with a four-point bending system (7). The researchers suggested that mechanosensitive osteocytes were responsible for the increase in IGF-I expression.
Beravenboer et al. studied the effect of exercise, with or without additional weight bearing (AWB), on the systemic and bone concentrations of IGF-I, IGFBP-3, and transforming growth factor beta (TGFβ) (16). There were no significant differences in the concentrations of IGF-I and IGFBP3 in the tibiae. In the humerus, IGF-I levels decreased in the group of rats that ran alone but increased in the rats that ran with AWB (16). The researchers believed that the rats running on a treadmill amassed more weight on the forepaws than on the hind paws. Although the results of the recent study showed a decrease in the IGF-I concentration in the humorous, our results and those of other studies demonstrated an increase in the IGF-I and IGF-I mRNA levels in the tibia. Also, the recent study reported an increase in IGF-I level only in the rats receiving exercise and AWB, where researchers argued that the number of repeated loads was the same in the animals performing exercises (such as endurance training) without or with AWB. The level of IGFBP-3 was measured only in the tibia and serum, and the results did not show significant differences between groups. It seems that some of our results differed from the findings of Beravenboer et al., which could be attributed to the difference in the method of gene expression analysis and the concentration of growth factors (16). Usually, an increase in gene expression precedes a rise in protein levels; therefore, a difference in the duration of exercise (30 to 60 minutes for eight weeks versus 15 minutes for six weeks) can also be another reason for the difference observed. Other factors that may explain the difference between studies are the speed and slope of the training program.
Osteogenic responses to mechanical stimulation are directly related to the generated strain, altering the expression and secretion of growth factors such as IGF-I, IGFBP-3, and TGFβ. Also, studies have demonstrated that TGFβ can stimulate the expression of IGF-I and IGFBP-3 (19).
The results of this study revealed that the combination of H2O2 and Vit D failed to make significant changes in the expression of IGF-I and IGFBP-3 compared to the H2O2-treated group. Moreover, the rats treated with H2O2+ E + Vit D did not show significant changes in IGF-I; however, changes in IGFBP-3 were remarkable (P ≤ 0.1).
In addition, 1α,25(OH)2D3 has been reported to stimulate the secretion of IGFBP-3 in human osteosarcoma cells (20, 21), which was correlated with the initial level of alkaline phosphatase (ALP) activity. It seems that IGFBP-3 levels directly correlate with ALP activity secondary to the interaction between vitamin D and its receptor on osteoblasts (20, 21). It has been indicated that IGFBP-3, with six VDR-related functional regions, is a primary target for 1α,25(OH)2D3, highlighting the interaction of VDR with a close promoter of the IGFBP-3 gene (21). These results confirm our previous findings, as well as the report of Shariati et al., noting an increase in the expression and secretion of ALP in the H2O2+ E + Vit D group (9).
One of the reasons for not observing a significant change in IGF-I in the H2O2 + E + Vit D group could be related to the fact that the concomitant supplementation of antioxidants (such as vitamin E) with exercise sometimes does not have the desired effects and may even adversely affect exercise-induced adaptation (22). According to our results, vitamin D supplementation and aerobic training may not have a synergistic interaction in modulating the expression of IGFBP-3 and IGF-I in bone tissue.
The limitations of this study include the lack of a healthy control group and not measuring some factors, such as the IGF-I receptor or bone mineral density. Accordingly, we suggest designing studies with healthy samples and evaluating the effects of different training methods on other bone growth factors.
5.1. Conclusions
The findings of this study indicated that the systemic attenuating effects of H2O2 on the local production of bone growth factors could affect the bone quality (density, stiffness, and fragility). Moreover, the modulatory impacts of exercise and vitamin D resulted in the overexpression of IGF-I and IGFBP-3 in the aerobic training group. Therefore, we recommend using this intervention to improve the metabolic performance of the bone tissue in disorders caused by the local overproduction of free radicals.