1. Background
Osteosarcopenic obesity (OSO) syndrome is associated with changes in body composition, including osteoporosis, sarcopenia, and increased fat tissue or fat distribution in the abdominal area and its penetration into bones and muscles (1). The simultaneous presence of sarcopenia and osteoporosis can affect each other because the loss of muscle mass and function can lead to the activation of catabolic processes in bone and vice versa (2).
Compared to men of the same age, older women tend to be more obese, have lower amounts of skeletal muscle mass, lower muscle strength and power, and lower muscle density (reflecting greater muscle fat penetration), which puts them at greater risk of physical dysfunction and disability (3). Physical activity and, in particular, resistance training are recommended as an intervention strategy to improve muscle strength and power, two factors that affect physical performance in older adults (4). Resistance training reduces fat percentage and improves bone mineral density (BMD) (5).
Several studies showed that resistance training for 12 weeks effectively increases skeletal muscle mass and improves the risk factors of OSO syndrome in older women (60 years old and above) (6). In addition to mechanical stress, some myokines secreted by muscles can also affect bone metabolism (7). Recently, insulin-like growth factor 1 (IGF-1) and fibroblast growth factor 2 (FGF-2) located at the muscle-bone interface have been recognized as two osteogenic factors (8).
Previous studies also showed that IGF-1 and FGF-2 stimulate bone formation in vitro and in vivo (9). The IGF-1 is a potent myoanabolic factor, and increased expression of IGF-1 with increasing muscle hypertrophy will likely increase the secretion and local abundance of IGF-1 at the muscle-bone interface.
In this pathway, muscle hypertrophy and bone anabolism are believed to be coupled through a paracrine signaling mechanism mediated by IGF-1. It has also been shown that muscle damage with eccentric contraction during exercise or with dystrophin deficiency increases the local release of FGF-2 and the circulating levels of FGF-2 (10).
2. Objectives
Given the results of the related literature on the positive effect of mechanical stress on muscle and bone growth, in this research, the Theraband was used to investigate the effect of muscle tension on muscle and bone interactions in addition to mechanical stress. Thus, the present research aimed to investigate the effectiveness of resistance training with the Theraband on IGF-1 and FGF-2 levels and their relationship with bone density in older women with osteosarcopenic obesity.
3. Methods
This study was a single-blind randomized clinical trial based on the Consolidated Standards of Reporting Trials (CONSORT) statement for randomized trials of non-pharmacological treatment. The present research has been registered in the ethics committee of the Research Institute of Physical Education and Sports Sciences (IR.SSRC.REC.1398.040) and the Clinical Trial Center of Iran with the code IRCT20190705044101N1.
3.1. Sample
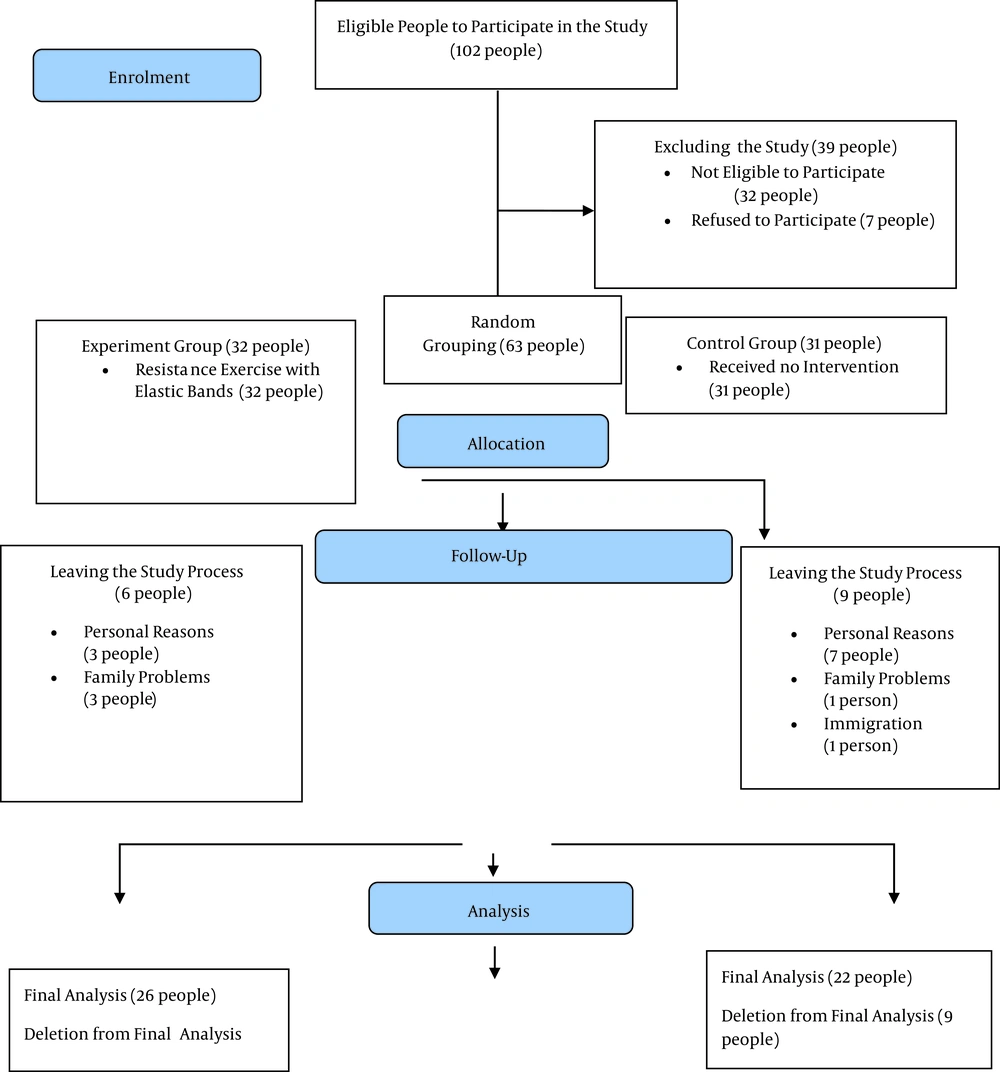
Using GPower software (version 3.1.9.2), the total sample size was calculated to be 48 people. The final sample size of 63 people was estimated after predicting the drop rate of 20%. A physician selected all eligible patients. Given Figure 1 (CONSORT flowchart) in this research, out of 102 initial referrals, 26 people in the experimental group and 22 in the control group completed the study. The samples were randomly divided into two groups: (1) control, and (2) resistance training. Eligible patients with osteosarcopenic obesity were monitored and selected according to the criteria proposed by the European Working Group on Sarcopenia in Older People, which can also be used in Iranian subjects. The subjects were selected using a dual X-ray absorptiometry tool energy (DEXA) with T-score ≥ 2.5-1 from L1-L4 or femur and lumbar spine, the age range of 60 - 80 years, body fat percentage > 32%, BMI > 30 kg/m2, and walking test (MWT) 10) ≤ 1 m/s2 (11). The exclusion criteria included the attending physician’s disallowance to continue sports exercises, performing parallel physical exercises, following a weight-losing diet of more than five kg in the last three months, and taking any medication that could affect bone density, fat tissue, or the hormonal system.
3.2. Training Protocol
Over two weeks before the start of the protocol, the training group performed resistance training with a yellow Theraband to get familiar with the training tools and the training environment and to correct the movements. In addition, in the first two sessions, patients were trained to control exercise intensity using the target number of repetitions (TNRs) and the OMNI resistance exercise scale (OMNI-RES) (12). Resistance training for all muscle groups was designed according to Di Liao’s protocol for three training sessions per week (13) (Figure 1). The control group samples did not participate in any nutrition or training protocol.
3.3. Sampling and Protein Measurement
After anthropometric measurements, a 5 cc blood sample was taken from the anterior brachial vein by the laboratory's blood sampling specialists two times at initial and 48 h after the last session. Then, the blood sample was centrifuged, and the serum sample was separated and kept at -70°C for analysis. The concentrations of IGF-1 (DIAMETRA kit, Italy) with a sensitivity of 0.58 ng/mL and FGF-2 (ESTABIOPHARM kit, China) with a sensitivity of 0.25 ng/L were measured by ELISA method.
3.4. Statistical Analysis
For statistical analysis, the Kolmogorov-Smirnov test was used for normality. Dependent samples t-test and Wilcoxon test were used for intra-group comparisons, and covariance analysis (ANCOVA) was used for inter-group comparisons. Also, Pearson correlation was used for correlation (SPSS 22, P ≤ 0.05).
4. Results
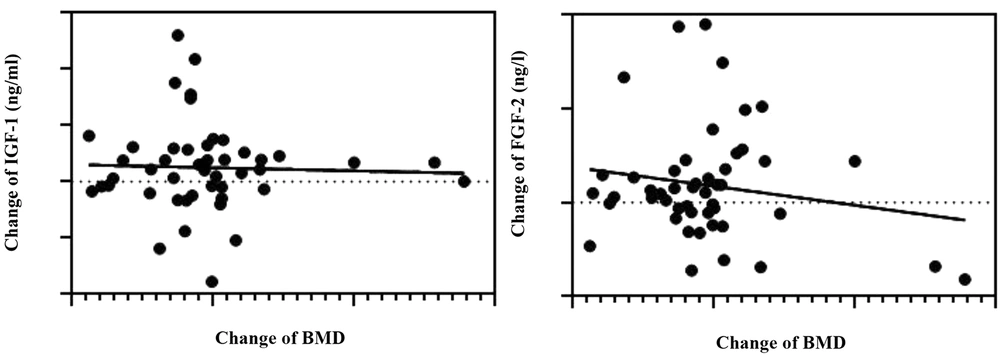
Based on the results of the present study, considering the inter-group comparisons, a significant difference was observed in IGF-1 (P = 0.042) after 12 weeks of resistance training with the Theraband, but the changes in FGF-2 (P = 0.444) were not significant. There was no difference in BMD in the control and training groups (P = 0.202) (Table 1). Also, no significant relationship was observed between the levels of IGF-1 (P = 0.240) and FGF-2 (P = 0.806) with BMD (Table 2, Figure 2).
| Variables | Control | Training | Intergroup P |
|---|---|---|---|
| Age (y) | 0.947 | ||
| Pretest | 64.05 ± 3.35 | 64.11 ± 3.81 | |
| Height (cm) | 0.812 | ||
| Pretest | 155.77 ± 4.14 | 155.59 ± 4.38 | |
| Weight (kg) | 0.001 | ||
| Pretest | 78.73 ± 7.52 | 81.66 ± 10.10 | |
| Posttest | 81.81 ± 8.03 | 81.87 ± 9.81 | |
| Intragroup P | 0.001 | 0.519 | |
| BMD (gr/cm3) | 0.202 | ||
| Pretest | 0.982 ± 0.117 | 0.956 ± 0.890 | |
| Posttest | 0.964 ± 0.119 | 0.960 ± 0.088 | |
| Intragroup P | 0.093 | 0.698 | |
| IGF-1 (ng/L) | 0.042 | ||
| Pretest | 63.08 ± 17.23 | 56.30 ± 16.47 | |
| Posttest | 62.89 ± 16.73 | 73.76 ± 22.49 | |
| Intragroup P | 0.956 | 0.013 | |
| FGF-2 (ng/mL) | 0.444 | ||
| Pretest | 270.52 ± 16.95 | 190.06 ± 66.77 | |
| Posttest | 286.21 ± 123.76 | 223.18 ± 93.44 | |
| Intragroup P | 0.45 | 0.003 |
| Factors | R | P-Value | |
|---|---|---|---|
| IGF-1 | BMD | -0.17 | 0.24 |
| FGF-2 | -0.04 | 0.81 | |
5. Discussion
The results of the present study showed that 12 weeks of resistance training with a Theraband caused a significant change in IGF-1 in older women with OSO; however, no significant difference was observed in FGF-2 and BMD. Significant changes in the functions of endocrine glands occur with increasing age. The levels of anabolic hormones, such as testosterone, growth hormone, and estrogen, decrease with age, which is associated with some changes in body composition and decreased performance associated with aging (14). The serum levels of IGF-1 negatively correlate with body composition, body metabolism, and age, which is more noticeable in women (15). Gender is another factor that can affect IGF-1 levels. Women lose one percent of their muscle mass yearly after menopause (16).
Studies have shown that resistance activity positively affects the factors that control serum IGF-1 levels, which increases the hormone level. The results of this research showed that 12 weeks of resistance training with the Theraband increased the levels of IGF-1 in the training group. Parakhouse et al. observed an increase in IGF-1 after eight months of resistance training in women aged 45 - 70 (17). Also, Fielding et al. found the same result with eight weeks of resistance training on older women over 65 years old (18).
The results of this research showed that 12 weeks of resistance training with the Theraband caused a significant increase in the levels of FGF-2 in the training group. The FGF-2 is actively secreted in muscle-bone connections and plays an important role in regulating cell proliferation (19). Clarke and Feeback demonstrated increased cytoplasmic secretion of FGF-2 with disrupted plasma membrane homeostasis caused by chronic mechanical stress on muscle cells (20). Muscle-derived FGF-2 affects muscle growth and regeneration by increasing the production of satellite cells. In line with the results of this research, Khadivi Borujeny et al. showed that eight weeks of resistance training increased FGF-2 in the training group of male Wistar rats (21). Hanssen et al. showed that the levels of FGF-2 in untrained healthy men increased after two weeks of strength training and decreased after 11 weeks (22). Kim et al., in their study on the effect of 12 weeks of resistance training on FGF-2 levels in the young (12 weeks old) and elderly (19 months old) mice, found that in the elderly control group, there was a significant increase in the levels of FGF-2 protein compared to the young control group in the soleus muscle. Also, a significant decrease was observed in the soleus muscle and tibia muscle of the elderly training group compared to the elderly control group (9). The observed difference may be related to training and measuring FGF-2 levels (blood serum in this study and muscle biopsy in other studies).
Research has shown that all sports activities are not bone-building and different types of sports activities affect bones and muscles differently (23). The results of the present study showed that 12 weeks of resistance training with a Theraband on the bone density of older women with osteosarcopenic obesity caused a significant increase in bone density; however, this effect was not significant compared to the control group. Consistent with our results, Hashemi et al. showed that 12 weeks of resistance training in water (two sessions per week) was not significant on the bone density of older women (60 - 75 years old) (24). Vanni et al. also reported that 28 weeks of resistance training has no significant effect on BMD in premenopausal women (25). However, Winters-Stone and Snow observed that resistance training for one year could increase BMD in postmenopausal women (26). Woo et al., during 12 months of Tai Chi and elastic band exercises on 90 men and 90 women aged 65 - 74, showed that Tai Chi exercises improved BMD in older women, but elastic band resistance exercises did not have a significant effect on BMD (27).
Despite having a long training period compared to the current study, our study obtained a similar result. It seems that in addition to the duration of the training period, another important factor in the effectiveness of the training program is the amount of mechanical stress resulting from the training. In programs that are accompanied by applying pressure for a suitable period, the results on BMD are significant (28). However, it appears that training such as Theraband, which affects muscle tissue more by applying tension instead of applying mechanical load, failed to significantly change BMD; however, it prevented the noticeable decrease observed in the control group.
Mechanical loading induces IGF-1 expression in skeletal muscle. Although IGF-1 protein from blood and bone circulation is physiologically necessary for bone metabolism, IGF-1 released from muscle may play a role in regulating bone regeneration for a large number of muscles in the body (29). In research in which female rats performed exercises on a treadmill for nine weeks, it was found that the trained rats had higher levels of serum IGF-1 and increased bone mass compared to the control group (30).
This study revealed no significant relationship between BMD and IGF-1 and FGF-2 levels. However, Kaji showed that the serum levels of IGF-1 have a positive relationship with BMD and the predictive factor of osteoporosis fractures (29). Different forms of training may be effective in stimulating increased levels of anabolic hormones and bone formation.
Studies have also shown that FGFs play an important role in regulating bone angiogenesis. Kigami et al. showed that FGF2 increases angiogenesis and enhances bone formation in rats with measured bone defects (31). The reasons for the lack of change in FGF-2 protein in bone concerning exercise may be as follows: (1) the stress applied to the bone by exercise was not sufficient to change the levels of osteoblast FGF-2 protein in the aging state; or (2) it may be related to the decrease in muscle level, as muscle and bone interact through FGF-2 (8). However, the underlying mechanisms of training-induced changes are difficult because training is a very complex process that simultaneously involves reciprocal responses and adaptations in several tissues and organs at the cellular and systemic levels (32).
5.1. Conclusions
It is a distinct feature of resistance exercises with Theraband that is a safe and simple implementation. Also, in addition to applying additional load, it affects the body’s homeostasis by creating tension in the muscles under resistance. This clinical trial also showed for the first time that resistance training with Theraband through the application of load and tension on muscle and bone caused the production of effective growth factors in the interaction of bone and muscle and even weight control and maintenance of bone mineral mass in elderly people with osteosarcopenic syndrome. Therefore, it can be used as an alternative model to traditional resistance training.