1. Background
Doxorubicin (DOX) is an anticancer drug with a high ability to reduce cell proliferation and diminish cancer progression (1). However, its cardiotoxic effect has limited its use (2). The heart is highly sensitive to DOX-induced oxidative damage due to its high mitochondrial mass and oxygen consumption, which are the primary sources of reactive oxygen species (ROS) (1-3). In response to excessive ROS generation, DOX damages the mitochondrial membrane and releases cytochrome c, causing apoptosis in cardiac cells. The release of cytochrome c regulates by the Bcl-2 family genes located in the inner mitochondrial membrane (4).
There are 2 groups of proapoptotic and antiapoptotic genes in the Bcl-2 family. Proapoptotic proteins (such as Bax) cause the release of cytochrome c from the mitochondria into the cytosol. Antiapoptotic genes (such as Bcl-2 and Bcl-XL) prevent the release of cytochrome c into the cytosol and inhibit apoptosis (5). Thus, the ratio of Bax to Bcl-2 is used to identify the inducing apoptosis in various cells (6).
One of the main causes of cardiotoxicity is the inducing oxidative stress by antitumor drugs. Thus, a suitable designed diet rich in antioxidants may decrease the risk of cardiotoxicity (7).
Saffron is one of the traditional spices widely used as a food flavoring and coloring agent in Iran. Saffron has 3 primary metabolites with therapeutic effects: Crocin (Cr; responsible for red color), picrocrocin (responsible for bitter taste), and safranal. Crocin is a water-soluble carotenoid with antioxidant and antitumor effects. Crocin has various beneficial effects, including antioxidant, anti-inflammatory, anticancer, and neuroprotective properties (8). Previous studies have shown that Cr prevents apoptosis in heart tissue by reducing the Bax/Bcl-2 ratio and inhibiting cytochrome c release from the mitochondria (9).
Some studies have revealed that antioxidants are not always desirable (10). Exercise can increase the activity of antioxidant enzymes and attenuate oxidative damage and apoptosis in the heart tissue (11, 12).
Previous studies have focused on nutritional factors and antioxidants to prevent the cardio-toxic effects of DOX. However, the effect of exercise as a strategy to reduce apoptosis in cardiac cells has received less attention. Aerobic exercise is a non-pharmacological therapy that attenuates DOX-induced cardiotoxicity and improves diastolic and systolic function (13).
Regular aerobic exercises cause adaptations in the cardiac muscle leading to an increase in the number and size of mitochondria (14). Previous studies have shown that exercise creates an antiapoptotic function in the heart (15). According to the recommendations of the American Sports Medicine Association, doing 30 minutes of moderate-intensity exercise at least 3 times a week is necessary to maintain body health (16). Many studies have demonstrated the beneficial effects of continuous aerobic training (CAT), especially in the long term, on blood pressure and heart rate (17).
Aerobic exercises and antioxidant consumption during DOX-based therapy may be a valuable approach to reducing cardiac cell death.
2. Objectives
This study aimed to investigate the simultaneous effect of CAT and Cr on DOX-induced apoptosis in the heart tissue of rats by evaluating the messenger RNA (mRNA) expression of Bax and Bcl-2 genes.
3. Methods
3.1. Animals
Fifty male Wistar rats weighing 200 - 250 g and 8 weeks old were used in this study. The rats were prepared from the Animal Reproduction and Breeding Center of Jundishapur University of Medical Sciences, Ahvaz. To get familiar with the new environment, the animals were kept in the new condition without any intervention for 1 week. The animals had free access to water and standard food and were maintained at 21 - 23°C, relative humidity of 50% - 60%, and a 12: 12 light-dark cycle. This study was approved by the Ethics Committee of Ahvaz Islamic Azad University (code: IR.IAU.AHVAZ.REC.1398.024). At the end of the experiment, the animals were euthanized under deep anesthesia, and their hearts were fixed in formalin for tissue processing (5 samples) or maintained in a freezer at -80°C (5 samples) for the evaluation of gene expression.
3.2. Experimental Design
The animals were randomly divided into the following groups:
(1) Control: received normal saline (8 weeks).
(2) DOX: received 2 mg/kg DOX (8 weeks).
(3) DOX + CAT: received DOX + CAT (5 days per week for 8 weeks).
(4) DOX + Cr: received DOX + 10 mg/kg Cr (8 weeks).
(5) DOX + Cr + CAT: treated with DOX, Cr, and CAT (8 weeks).
Doxorubicin (EBEWE, Belgium) was diluted in normal saline at a dose of 2 mg/kg and injected intraperitoneally (7) on Fridays at 10 AM. Each injection was given 48 hours after the last training session of the previous week and 24 hours before the first training session of the following week.
Crocin powder (Sigma-Aldrich) was dissolved in normal saline and administered by gavage (10 mg/kg). It had fed to subjects at the same time as the CAT (7).
3.3. Maximum Running Speed Test
Animals run on a treadmill at a speed of 10 m/min. Every 2 minutes, the speed was increased by 1.7 m/min until paralyzed the animals. If the animal touched the end channel of the treadmill 5 times within a minute, it was considered a measure of retardation (18).
3.4. Training Design
The exercise instruction consisted of running on an electronic treadmill for rodents in a continuously and increasingly manner based on previous studies (18). The training was performed 5 days per week for 8 weeks (60 minutes in each session). The first week of training was performed with an intensity of 40% of the maximum speed. From the second to the fourth week, the speed reached 50% - 55% of the maximum speed, and from the fifth until the eighth week, it reached 60% of the maximum speed. All training sessions started with a 5-minute warm-up and a 5-minute cool-down at a speed of 7 m/min.
3.5. Real-time Polymerase Chain Reaction
The RNA of the hearts was isolated using an RNeasy kit and converted to complementary DNA (cDNA) using a cDNA kit. The cDNA amplified in the polymerase chain reaction (PCR) contained primers (Table 1) and SYBR Green. The following 45-cycle program had used to amplify the PCR: 95°C for 10 seconds, 95°C for 15 seconds, 60°C for 20 seconds, and 60°C for 20 seconds. In this research, the GAPDH gene was used to normalize target genes. The relative expression of genes was investigated using the 2-ΔΔCT method.
| Target Genes | Control |
|---|---|
| GAPDH | Forward: 5′-GCAAGAGCACAAGAGGAAGA-3′ |
| Reverse: 5′-ACTGTGAGGAGGGGAGATTC-3′ | |
| Bax | Forward: 5′-GCTGGACATTGGACTTCCTC-3′ |
| Reverse: 5′-ACCACTGTGACCTGCTCCA-3′ | |
| Bcl-2 | Forward: 5′-GGATGCCTTTGTGGAACTGT-3′ |
| Reverse: 5′-TCACTTGTGGCCCAGATAGG-3′ |
Primer Sequences
3.6. Terminal Deoxynucleotidal Transferase-Mediated Biotin-Deoxyuridine Triphosphate Nick-End Labeling Staining
The percentage of apoptosis was determined using a terminal deoxynucleotidal transferase-mediated biotin-deoxyuridine triphosphate nick-end labeling (TUNEL) assay kit (Roche, Germany). In brief, deparaffinized sections were permeabilized and treated with proteinase K for 30 minutes at 37°C. Then, the sections were treated with 50 μL of TUNEL-reactive solution for 60 minutes at 37°C. After washing with phosphate-buffered saline (PBS), the sections were treated with 50 μL of converter solution for 30 minutes at 37°C. After washing with PBS, the sections were incubated in diaminobenzidine tetrachloride solution for 20 minutes and evaluated under a light microscope.
3.7. Statistical Analyses
We used SPSS version 21 (SPSS Inc, Chicago, Ill, USA) to analyze the data. The Shapiro-Wilk test was used to investigate the normal distribution. Then, a post-hoc pair-wise comparison was made using the Bonferroni technique. P values less than 0.05 were considered statistically significant.
4. Results
4.1. Real-time
A one-way analysis of variance (ANOVA) showed that the expression levels of Bax and Bcl-2 genes and the Bax/Bcl-2 ratio in the heart tissue were significantly different between the studied groups. Therefore, the Bonferroni post hoc test was performed.
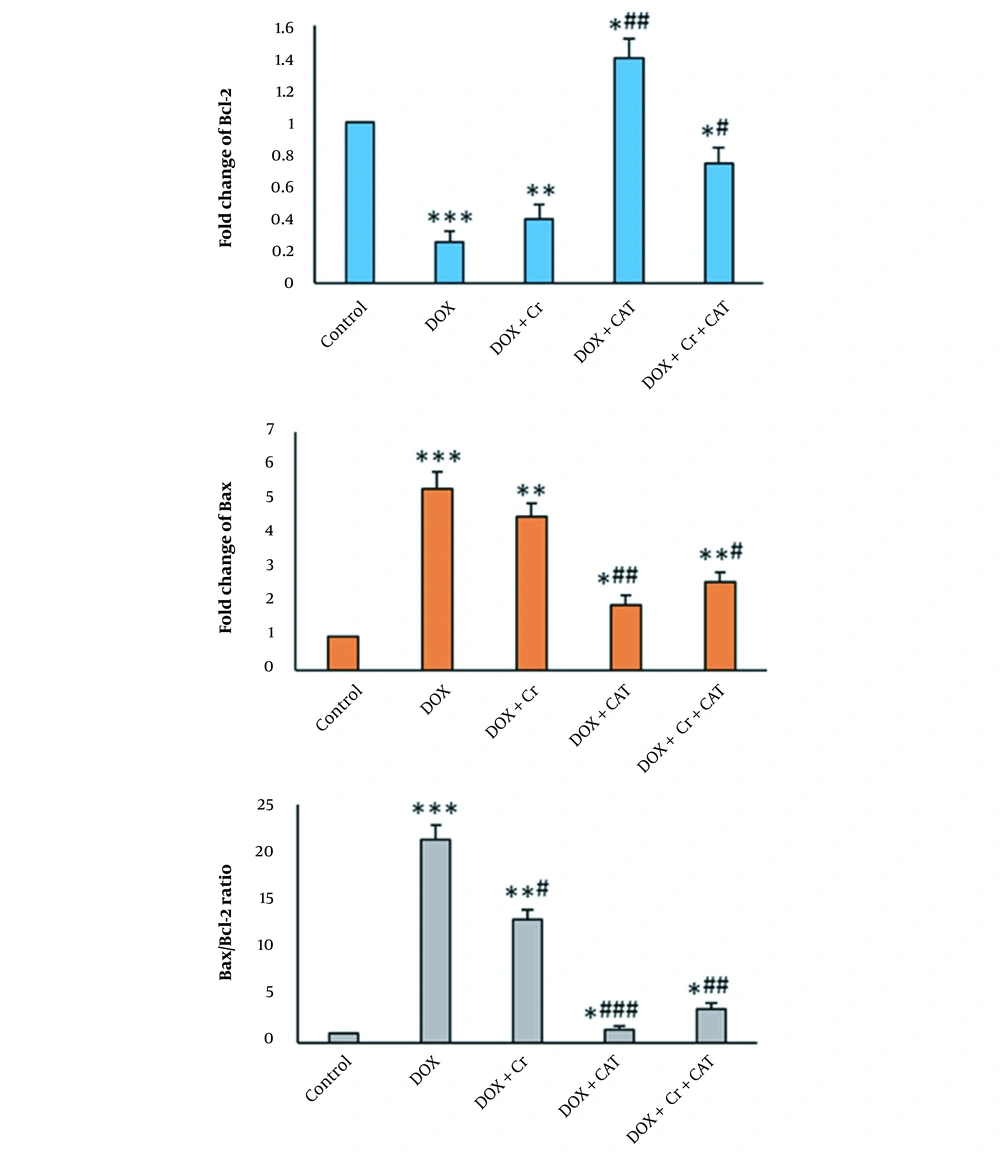
In the DOX group, the expression of the Bax gene in the heart tissue of rats significantly increased (P < 0.001). Administration of Cr alone, undergoing CAT alone, and their combination significantly reduced Bax expression in the heart tissue of DOX-intoxicated rats (Figure 1). The expression of the Bcl-2 gene in the heart tissue of rats significantly increased (P < 0.001) in the DOX group. Administration of Cr and undergoing CAT alone and at the same time significantly enhanced Bcl-2 gene expression in the DOX-induced cardiotoxicity in rats (Figure 1).
The ratio of Bax/Bcl-2 in the DOX-intoxicated rats was considerably enhanced compared to the control (P < 0.001). Administration of Cr alone and undergoing CAT alone or in combination significantly reduced the Bax/Bcl-2 ratio in the heart tissues of DOX-intoxicated rats. The decrease was significantly lower in the Bax/Bcl-2 ratio in the combination of the Cr and CAT group than in the treated Cr alone group. DOX + CAT decreased Bax/Bcl-2 ratio was higher than the DOX + Cr (P-value) and DOX + Cr + CAT groups (Figure 1).
4.2. Terminal Deoxynucleotidal Transferase-Mediated Biotin-Deoxyuridine Triphosphate Nick-End Labeling Staining
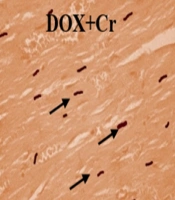
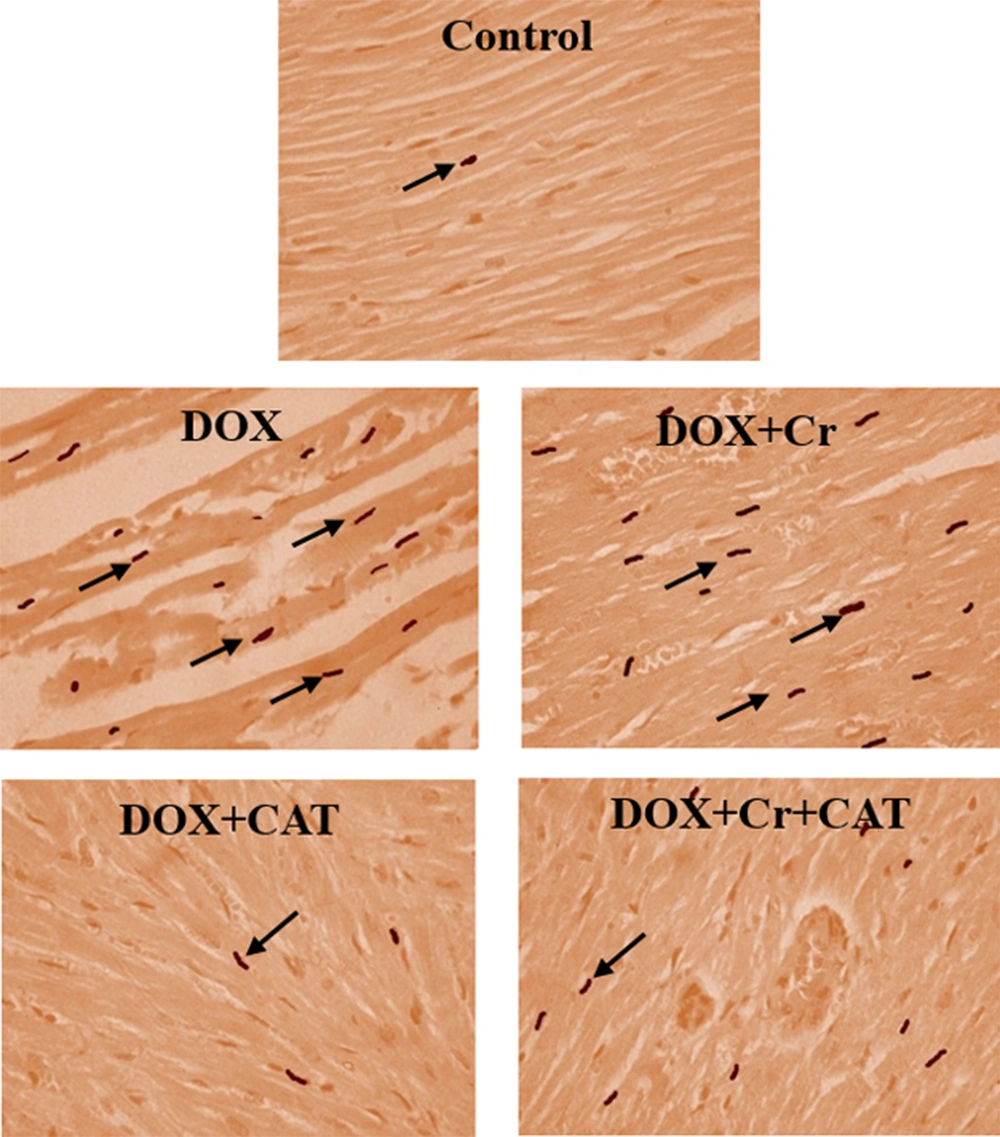
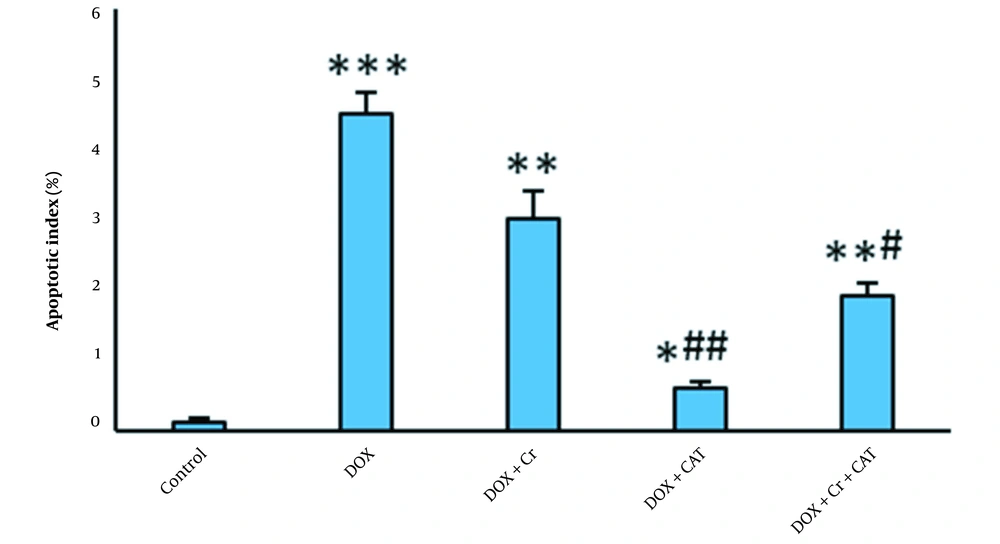
The percentage of TUNEL-positive cells in the DOX-intoxicated rats significantly increased compared to the control group (P < 0.001). Administration of Cr or undergoing CAT alone or simultaneously significantly reduced the number of TUNEL-positive cardiac cells in DOX-intoxicated rats.
The percentage of TUNNEL-positive cells of the Cr + CAT group was significantly lower than the group treated only with Cr. CAT had more impacts on decreasing the apoptotic index of the DOX-intoxicated hearts than the Cr and Cr + CAT groups (Figures 2 and 3).
5. Discussion
In this study, the effect of CAT and Cr against apoptosis caused by DOX in heart tissue was investigated. As mentioned in the results, CAT decreased the ratio of Bax to Bcl-2 gene expression, indicating its antiapoptotic effects. This finding is consistent with previous studies that revealed that exercise increases the body’s resistance to apoptosis by reducing the ratio of Bax/Bcl-2 expression. Razavi Majd and Ghahramani showed that aerobic training for 3 months led to a significant decrease in Bax gene expression (19). The endurance of physical activity increases the expression of Bcl-2 proteins in rats with high blood pressure (20). In another research, regular high-intensity activities decreased the ratio of Bax to Bcl-2 in the heart tissue (21). Ascensao et al. showed that DOX caused mitochondrial dysfunction, oxidative damage, histopathological damage, and apoptosis in the heart tissue of rats (22). In their study, endurance training could attenuate DOX-induced oxidative stress, increasing the Bax/Bcl-2 ratio and elevating caspase activity (22). Due to the high mitochondrial volume and low antioxidant defense, the heart is vulnerable to the increased oxidative stress caused by DOX. In our previous study, we showed that CAT reduced oxidative stress induced by DOX in the heart tissue of rats (23).
Doxorubicin disrupts mitochondrial bioenergetics, and aerobic exercise restores it by positive regulation of Krebs cycle enzymes and ATP synthase activity, which can reduce the oxidative effects of DOX. Oxidative stress can cause apoptosis via modulating Bcl-2 family genes (24). Thus, antioxidants can prevent oxidative damage and apoptosis of DOX in the heart tissue.
As mentioned in the results, Cr decreased apoptosis of the cardiac cells following the use of DOX, which is in line with previous studies. Razavi et al. found that Cr decreased the ratio of Bax/Bcl2 expression of diazinon-induced cardiotoxicity and reduced apoptosis in rats (25). Razmaraii et al. found that Cr improves apoptosis caused by DOX in rats (8).
Other studies have also reported beneficial impacts of Cr against DOX-induced cardiotoxicity. In the study conducted by Chu et al., Cr was found to have a protective effect on DOX-induced cardiotoxicity both in vitro and in vivo (26). Motlagh et al. reported the protective effects of Cr on DOX-induced cardiotoxicity (27).
This study could not reveal the exact mechanism of CAT and Cr on DOX-induced apoptosis. However, the downregulation of Bax or upregulation of Bcl-2 indicates that suppression of the intrinsic apoptosis signaling pathway is involved in the protective action of CAT or Cr against DOX-induced cardiotoxicity.
On the other hand, CAT or Cr may reduce apoptosis via activation of antioxidant defense of cardiac cells in the DOX-intoxicated rats. Previous studies have revealed that antioxidants inhibit DOX-induced apoptosis (28).
5.1. Conclusions
Continuous aerobic training and consumption of Cr partially protect the heart against DOX by suppressing apoptosis in rats. Interestingly, undergoing CAT for 8 weeks had a more pronounced effect than other experimental groups. Future studies are required to determine the exact mechanism of CAT or Cr on cardiotoxicity.