1. Background
Gastric cancer (CG) is one the most common cancers worldwide, which is usually diagnosed at late stage since it is asymptomatic in early stages (1, 2). Tumor resection and surgery are the only effective strategies to treat it, while chemotherapy has proven ineffective in this regard (3). Molecular targeted therapies of cancers have been proposed in recent decades as alternative treatment methods. However, it is necessary to identify the involved molecular pathways before designing novel and specific therapeutics methods for each cancer (4).
Long non-coding RNAs (lncRNAs) are defined as lengthy transcripts (larger than 200 nt) lacking protein-coding capacity (5). The involvement of lncRNAs in broad spectrum of biological processes such as cell cycle, growth, differentiation, and apoptosis has been reported (6). Aberrant expressions of lncRNAs have been found in a variety of human tumors, suggesting that these non-coding transcripts are potential biomarkers of cancers (7). The HOX antisense intergenic RNA (HOTAIR) is an oncogenic lncRNAs playing a critical role in promotion, progression, and invasion of variety of human cancers (8). The involvement is well-defined and established (9). It has been reported that silencing of HOTAIR exerts an anti-tumor effect on gastric cancer and potentially inhibits cell growth (10). Epithelial-mesenchymal transition (EMT) is a cellular process whereby epithelial cells lose their characteristics and obtain mesenchymal phenotypes. Following EMT, epithelial cells lose cell-cell joints, polarity, and epithelial markers, and then non-motile epithelial cells gain cell motility, a spindle-cell shape, and presenting mesenchymal markers (11). Epithelial-mesenchymal transition trigger the cancer metastatic cascade through different signaling pathways (12). Claudins are structural and functional elements of tight junction playing an important role during EMT (13). Dysregulation expression of claudins has been reported in several human tumors, and the pattern depends on claudins isoform or cancer type (14). Loss of function of these proteins affect the cell-cell adhesions and prepare the cellular micro environment toward malignant transformation (15). Fibronectin (FN) is an abundant extracellular matrix (ECM) glycoprotein that functions during tissue repair (16). Altered expression of fibronectin in various human tumors proposed them as promoters of cancer progression and metastasis (17).
2. Objectives
Accordingly, the present study aimed to investigate the effect of HOTAIR long non-coding RNA on expression levels of the fibronectin-1 and claudin-4 adopting siRNA strategy.
3. Methods
The present study was extracted from M.Sc. thesis written by S.A., which was approved by Ethical Committee of Islamic Azad University Tehran Medical Sciences, Research Affairs, Tehran, Iran. University (codding number and code of ethics: IR.IAU.PS.REC.1401.122).
3.1. Cell Lines and Culture Condition
The MKN45 cell line was used as cellular model of human gastric adenocarcinoma. Cells were purchased from national cell bank of the Pasteur Institute, Tehran, Iran. The cells were cultured in Dulbecco's modified eagle medium (DMED) (Life Technologies, USA) supplemented with 10% heat inactivated fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin antibiotics (Life Technologies, USA). The cells were maintained at 37°C in a humidified atmosphere of 5% CO2. The MKN45 cells were sub-cultured every 2 - 3 days.
3.2. HOTAIR siRNA Transfection
HOTAIR specific-siRNA was purchased from (Gene Pharma, China) with product ID number: 100,124,700. The siRNA sequences were: HOTAIR:5'UAACAAGACCAGAGAGCUGUU-3', negative control: 5'-TTCTCCGAACGTGTCACGT-3'.
The MKN45 cells were plated in 12-well plates (12 × 104 cell/well) and transfected by 50 ng siRNA duplexes through lipofectamine 2000 (Invitrogen, USA) according to manufacturer's protocol. The cells following the transfection process with no siRNA was considered as mock-treated cells. The cells were subjected to RNA extraction after 48 h post-treatment with siRNA.
3.3. RNA Extraction and cDNA Synthesis
Total RNA was extracted from MKN45 cells by RNX-plus solution using product guideline (SinaClon, Iran). The RNAs were treated with DNaseI (Thermo Fisher Scientific, USA) to prevent genomic DNA contamination. PrimeScriptTM RT kit (Takara, Japan) was used to synthesize cDNA. The primers used in this study are presented in Table 1.
| Primer | Forward Sequence 5'-3' | Reverse Sequence 5'-3' | Amplicon Size (bp) |
|---|---|---|---|
| HOTAIR | AGGCCCTGCCTTCTGCCT | TGCTCTCTTACCCCCACGGA | 147 |
| FN1 | GCAACTCTGTCAACGAAGG | TTCCAAAGCCTAAGCACTG | 147 |
| CLDN4 | CATCATCAGCATCATCGTGG | GGCACTATCACCATAAGGC | 153 |
| GAPDH | GTGAACCATGAGAAGTATGACAAC | CATGAGTCCTTCCACGATACC | 123 |
3.4. Real Time Polymerase Chain Reaction
Real-time-PCR was performed using SYBR green Premix Ex TaqTM II kit (Takara, Japan), and the data were analyzed using Livak equation (2-∆∆Ct method).
3.5. Statistical Analysis
Statistical analysis was carried out using Prism® 8 software (GraphPad Software, Inc, La Jolla, CA, USA), and the data were analyzed using one-way analysis of variance followed by Newman-Keuls multiple comparison test or student's t-test. Differences among groups were considered statistically significant when P < 0.05. The data were shown as mean ± standard deviation.
4. Results
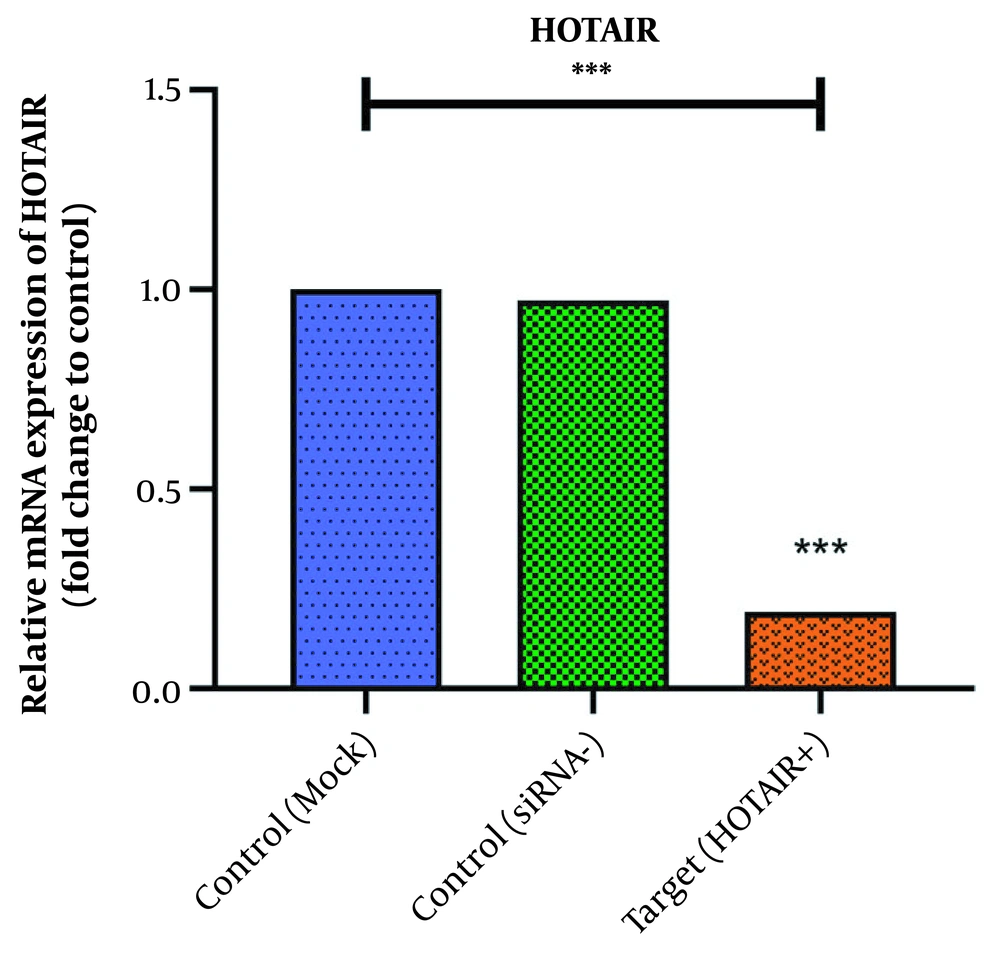
To evaluate the efficacy of the employed siRNA, the expression of HOTAIR was measured using real-time PCR. As indicated in Figure 1, the expression level of HOTAIR was high in MKN45 cancer cells. After 48h transfection with its specific siRNA, the expression levels of this lncRNA were significantly reduced (P-value < 0.001). The fold change was calculated as 0.191 ± 0.039. This data showed that the siRNA had acted successfully.
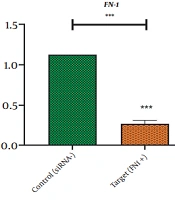
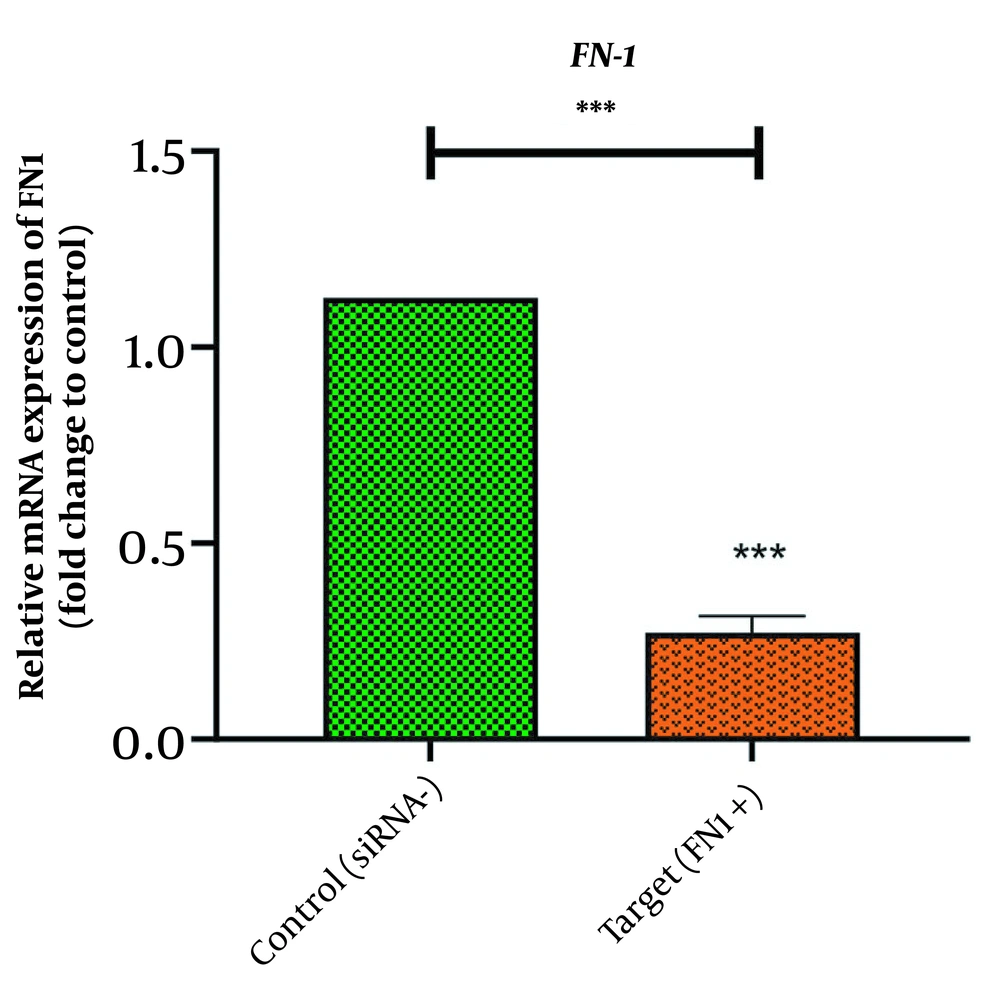
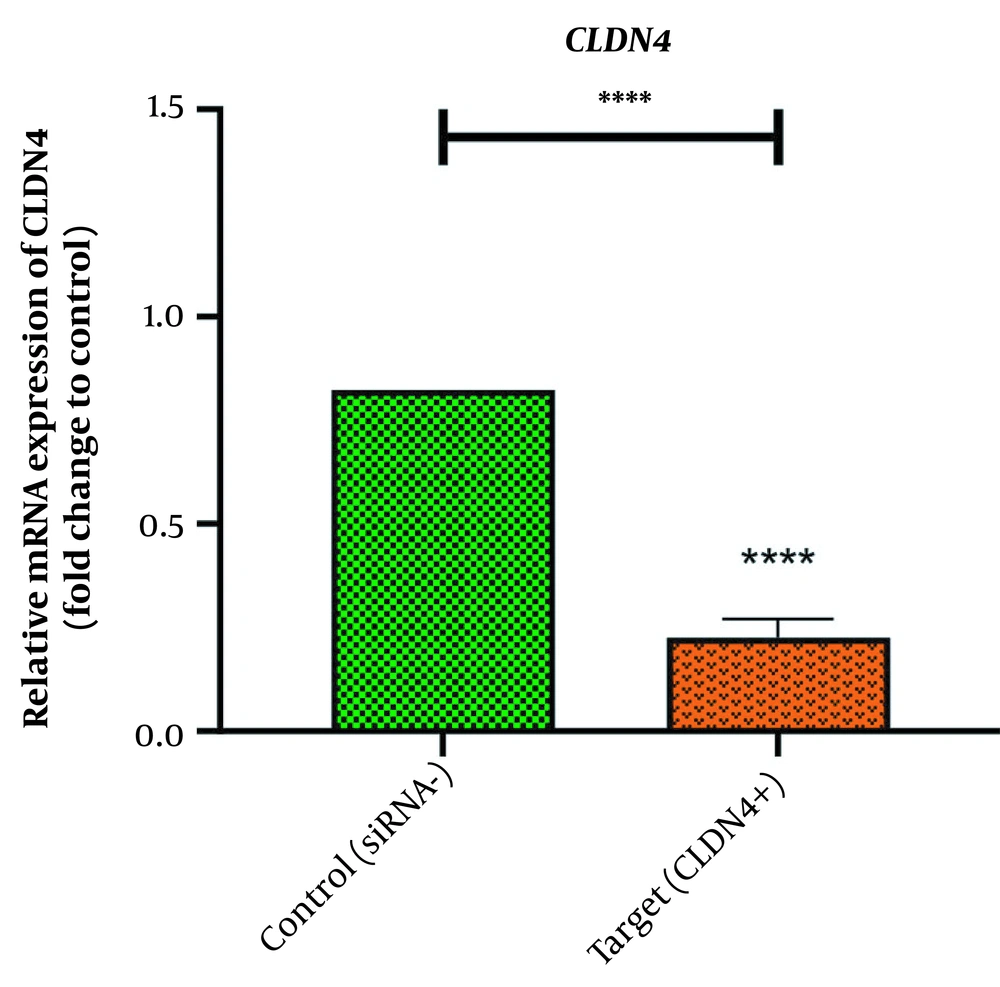
In the same vain, the expression levels of CLDN4 and FN1 were well-quantified with real-time PCR in order for addressing the possibility of the changes, triggered by silenced HOTAIR, in the expression levels of these EMT genes. Our observation revealed that FN1 and CLDN4 were remarkably transcribed in MKN45 cell lines (Figures 2 and 3). However, the treatment with HOTAIRs siRNA downregulated the expression of FN1 (Figure 2) and CLDN4 (Figure 3). The CLDN4 and FN1 fold-changes were 0.225 ± 0.0451(P-value < 0/0001) and 0.271 ± 0.0432 (P-value < 0/001), respectively. This meant that FN1 gene was downregulated three times, and CLDN4 gene was reduced four times after transfection.
5. Discussion
To our knowledge, the present study was the first to show the effect of HOTAIR silencing on expression levels of the two EMT markers, called fibrinectin-1 and claudin-4, in cellular model of gastric adenocarcinoma (MKN45). The obtained data indicated that both markers were significantly downregulated after silencing of HOTAIR.
The role of EMT in occurrence of cancer invasion has been repeatedly confirmed by previous studies suggesting that this pathway is a molecular target of cancer remedy (18). It is worth noting that cancer-associated mortalities are due to metastatic tumors rather than primary ones (19).
Fibrinectin-1 (FN1) plays an important role in cell adhesion, cell growth, migration, and differentiation (20). This glycoprotein mediates the interaction between cells and extracellular matrix (21). High expression of fibrinectin-1 has been observed in several cancers, such as gastric and thyroid cancers, suggesting that it may play a role during cancer progression and invasion (20, 22). Silencing of fibrinectin-1 results in cell growth arrest and proliferation (23). In a study by Liu et al., the knockdown of HOTAIR was discovered to prevent E-cadherin reduction and inhibit fibronectin upregulation, which suggested that HOTAIR triggered the EMT program (24).
Similarly, Padua Alves et al. demonstrated that the fibronectin expression level was downregulated, while HOTAIR was silenced in breast cancer (25). Babapour et al. determined that silencing the long non-coding RNA HOTAIR suppressed EMT in AGS gastric cancer cell line by downregulating fibronectin 1 (26). HOTAIR has also been detected to reduce transcription level of the metastasis-associated genes like fibronectin (27).
Claudins are located in tight junctions of epithelial cells regulating the exchange of particles in the intercellular space between these cells (28).
Altered expression of claudins has been documented in various cancers (29). There is a growing body of evidence that claudins have considerable clinical significance and promising utility in diagnosis, prognosis, and treatment of human cancers (30). Dysfunctioning of claudins and other tight junction proteins in cancer has been recognized by previous studies as a mechanism for the loss of cell adhesion, an important step in the progression of cancer to metastasis (31). Claudins can act as either oncogene or tumor suppressors depending on claudins isoforms and cancer type (32). Among different isoforms of claudins, CLDN4 has not received enough research attention. Increased expression of CLDN4 has been found to be associated with tumor invasion and metastasis in gastric cancer (33). Silencing claudins induces apoptosis in several cancers (34). According to Babapour et al., silencing the long non-coding RNA HOTAIR suppresses EMT in AGS gastric cancer cell line by downregulating claudin-4 (26).
5.1. Conclusions
In sum, our novel finding confirmed that HOTAIR silencing downregulated the expression levels of both important markers of EMT genes, namely CLND4 and FN1. However, it was recommended that further investigations should be carried out in this regard.