1. Background
Diabetes is one of the most common metabolic diseases (1). The common feature of metabolic diseases is the chronic activation of the inflammatory system (2). One of the most prevalent complications is diabetic peripheral neuropathy (DPN), caused by diabetes and a chronic increase in blood sugar. It is a disorder in the peripheral nerves of individuals with diabetes (3), leading to painful and non-painful symptoms (4). It has been determined that neuropathic pain occurs in 50% of people with diabetic peripheral neuropathy (5). Neuropathic pains are associated with the destruction or dysfunction of the central or peripheral nervous system (6). Hyperglycemia induces oxidative stress in neurons and activates many biochemical pathways that regulate gene expression in the reactions leading to neurological disorders (7).
Previous research in humans and animals has shown increased neuropathic pain due to inflammatory responses (8). Based on the research, the toxicity resulting from hyperglycemic conditions causes an increase in inflammatory mediators such as cytokines, including TNF-α, leading to an increase in other pro-inflammatory cytokines (9). The sensitivity of nerve receptors increases with the inflammatory activity caused by diabetes (10).
Among the therapeutic methods recommended for treating diabetic neuropathy, physical activity can be effective with a non-invasive and low-cost approach (11). Regular aerobic training with blood sugar control reduces oxidative stress in diabetic patients (12) and ultimately reduces neuropathic pain (13). On the other hand, researchers have shown that despite having various medicinal properties such as anti-aging, anti-oxidant, anti-cancer, and anti-inflammatory effects, melatonin also has anti-diabetic features. It achieves this by regulating diverse mechanisms in cells (14). Also, the effect of melatonin as an analgesic and anti-anxiety substance has been proven in animals and humans (15).
2. Objectives
Many studies have been conducted on neuropathic pain, especially painful diabetic neuropathy (PDN) (16). Neuropathic pain is difficult to treat, and few patients experience complete relief (13). This study aimed to study and analyze the effect of aerobic training in six weeks, along with melatonin consumption, on TNF-α expression in the neuroinflammation signaling pathway in the sensory part of the spinal cord of male rats with type 1 diabetes.
3. Methods
We obtained 25 male Wistar rats, aged 8 weeks with an average weight of 204 ± 11.3 g, from the Animal Breeding Laboratory at Jundishapur University of Ahvaz, Department of Medical Sciences. The rats were housed in standard polycarbonate cages in groups of four animals. The temperature was regularly monitored and remained within the range of 20°C to 24°C. The lighting system was also automatically set to a 12-hour light and 12-hour dark cycle. Rats had free access to water and food. One week later, they were randomly divided into 5 groups (n = 5), including diabetic, melatonin diabetic, training diabetic, melatonin and training diabetic, and control. The present research was approved by the Ethics Committee of the Islamic Azad University of Ahvaz, with the code of IR.IAU.AHVAZ.REC.1401.107.
3.1. Induction of Diabetes
Diabetes was induced by administering an intraperitoneal injection of streptozotocin solution (STZ Sigma Chemical Co, St. Louis, MO) after 12 hours of fasting. The solution was buffered with pH 4.5 and dissolved in 0.05 M citrate. Non-diabetic rats received the same volume of 0.05 M citrate, which was intraperitoneally buffered with a pH of 4.5. Blood glucose levels were measured using a glucometer (manufactured by Roche Co., Germany) from the rat’s tail vein. To be considered diabetic, the blood sugar level must be above 250 mg/dL. To ensure that blood sugar levels did not recur, they were measured weekly throughout the training period until completion (17).
3.2. Melatonin Injection
Two weeks after inducing diabetes and confirming diabetic neuropathic pain, the aerobic training protocol was initiated. To prevent the development of diabetic neuropathic hyperalgesia, melatonin (10 mg per kg of body weight) dissolved in a saline solution containing ethanol was injected intraperitoneally daily for six weeks in both the melatonin diabetic and melatonin training diabetic groups (18).
3.3. Pain Perception Test
Before the induction of diabetes, to adapt to the behavioral test, the animals were exposed to the test for three days (twice for the test). Two weeks after the induction of diabetes, neuropathic pain behavioral test was performed to confirm neuropathic pain in the groups (19).
3.4. Hot Plate Test
We utilized the hot plate model MH-9500 manufactured by Sanat Azma Company to evaluate thermal hypersensitivity. The temperature was set between 50°C and 54°C.
Licking, lifting, or shaking a leg was considered a sign of pain, and the animal was immediately removed from the device. The paw or tail withdrawal latency time, which is the time interval from when the rats were placed on the hot plate until they exhibited a pain response, was measured in three stages, each lasting for several seconds. Measurements were taken at 5-minute intervals for different groups, and the duration of the delay was determined by calculating the average of the records. A 30-second duration was established as the criterion for determining the rat’s non-reaction to the hot plate (cut-off time).
3.5. Aerobic Training Protocol
The duration of the aerobic exercise protocol was 6 weeks. Chae et al.’s aerobic training protocol was employed (20). Aerobic exercise was performed between 4:00 pm and 6:00 pm.
3.6. Sampling and Assessment Method
After 48 hours of the last experimental session, the rats were anesthetized by administering Ketamine (90 mg/kg) in combination with xylazine (10 mg/kg) intraperitoneally.
The target area was identified following sterile conditions and the method of Gelderd and Chopin (1997) (21). The lower part of the spine was then separated by cutting, and the posterior region of the spinal cord was collected as samples. These samples were stored in a freezer at - 80°C until histological analysis was conducted.
3.7. Real-time Polymerase Chain Reaction
To extract total RNA, approximately 55 mg of spinal cord tissue was homogenized at a ratio of 1:10 using the QIAzol Lysis Reagent kit. To remove protein fragments, the sample was centrifuged at 12,000 g for 10 minutes at 4°C. Subsequently, a chloroform solution was added to the sample at a ratio of 1:0.5 and vigorously shaken for 15 seconds. The centrifugation was performed for 15 minutes, applying 12000 g to the solution at 4°C to extract the mineral components and aqueous fractions. The portion containing RNA was mixed with an isopropanol solution at a ratio of 1: 0.5, then left at room temperature for ten minutes before being centrifuged for 10 minutes. The RNA pellet was washed with an ethanol solution at 4°C and 12000 g. After washing, 20 µL of RNase-free water was added. The concentration of RNA was calculated, and following the instructions provided by the manufacturer (Eppendorf, Germany) for the 260 to 280 ratio, an optimal concentration range of 1.8 to 2 was determined.
To perform single-stranded cDNA synthesis, we used the RT enzyme-reverse transcription (Fermentas) and primer enzyme (Oligo dt MWG-Biotech, Germany) following the appropriate protocol. The RT-qPCR method was employed to measure TNF-α expression levels. Primers (for the TNF-α gene and GAPDH as internal control) were designed by referring to sequences in the NCBI database (Table 1). All PCRs were performed using the PCR master mix from Applied Biosystems and SYBR Green on the ABI Step One machine (Applied Biosystems, Sequence Detection Systems, Foster City, CA), following the manufacturer’s instructions. For all real-time PCR experiments, 40 cycles were performed with the following temperature settings: 94°C for 20 seconds, 58 - 60°C for 30 seconds, and 72°C for 30 seconds. Additionally, the control gene used was GAPDH. The gene expression ratio of the study was analyzed using the CT threshold cycle comparative technique. The reference gene was used to normalize the expression of TNF-α, and the data were analyzed using the R = 2-ΔΔCT formula. The calibrator represented the expression of the control group genes.
| Genes | Primer Sequences (5’ – 3’) |
|---|---|
| TNF-α | |
| Forward | GAGAAGATGATGTGAGTGTGAGG |
| Reverse | GAGATGTGGAAATGGCAGAGGA |
| GAPDH | |
| Forward | GACAACTTTGGCATCGTGGA |
| Reverse | ATGCAGGGATGATGTTCTGG |
3.8. Data Analysis
The Kolmogorov-Smirnov test was used to determine the normality of the data. To analyze research assumptions using SPSS-22 software, a one-way analysis of variance and Tukey’s post hoc tests were conducted, and the data were analyzed at a significance level of P ≤ 0.05.
4. Results
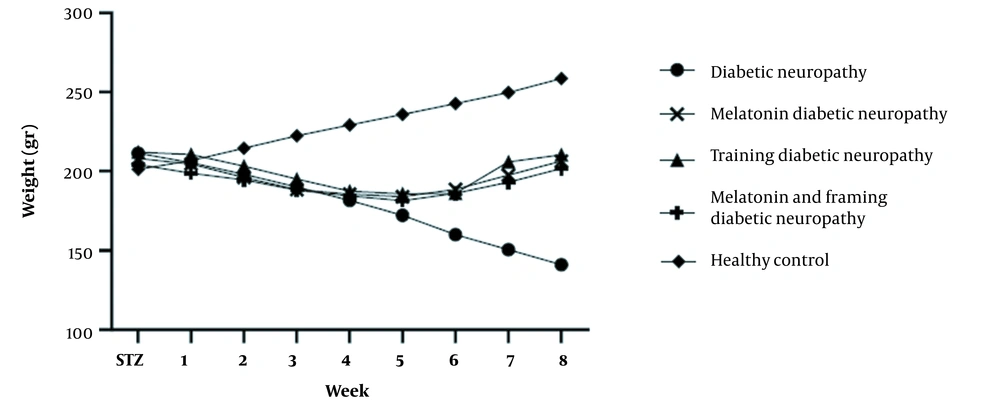
The mean ± SD of weight, glucose levels, and hot plate test results are presented in Figures 1 to 3. Figure 1 shows no significant difference in the initial weight of rats between various groups. Nevertheless, changes in the animals’ weight were significantly lower in the diabetic groups than in the control group (P < 0.05).
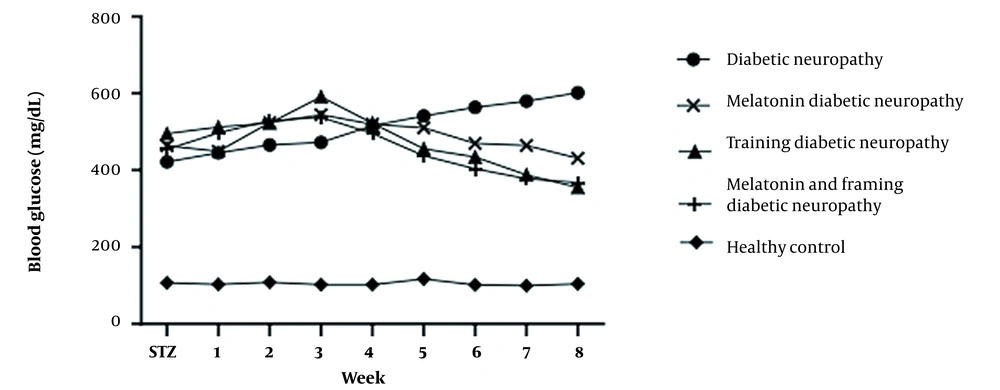
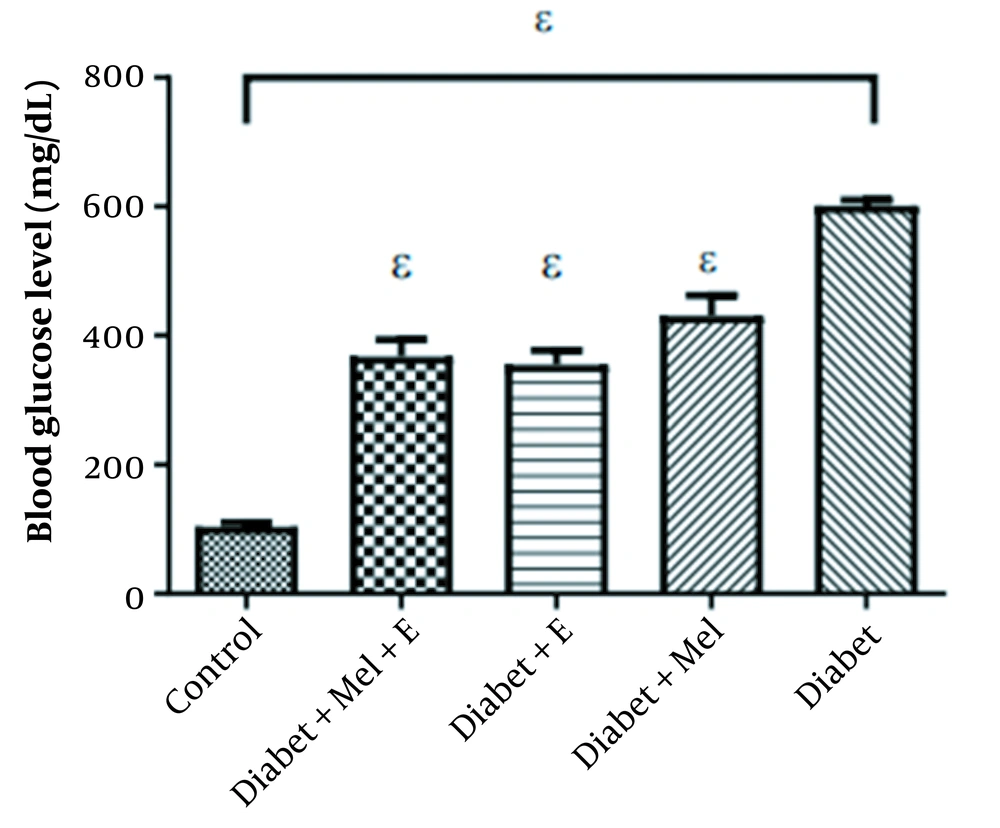
The induction of diabetes (Table 2) significantly increased blood glucose levels in the diabetic groups and showed a significant difference compared to the control and other groups (P < 0.0001). No significant difference was observed among other groups. Although exercise and melatonin decreased blood glucose, this reduction could not restore blood glucose to the level of the control group (Figure 3).
| Groups | TNF-α | Blood Glucose (mg/dL) |
|---|---|---|
| Control | 1.00 ± 0.43 | 104.25 |
| Diabetes | 3.74 ± 0.68 | 601.88 |
| Diabetes - Melatonin | 1.63 ± 0.49 | 430.88 |
| Diabetes - Exercise | 1.88 ± 0.60 | 355.25 |
| Diabetes - Exercise + Melatonin | 1.08 ± 0.42 | 367.88 |
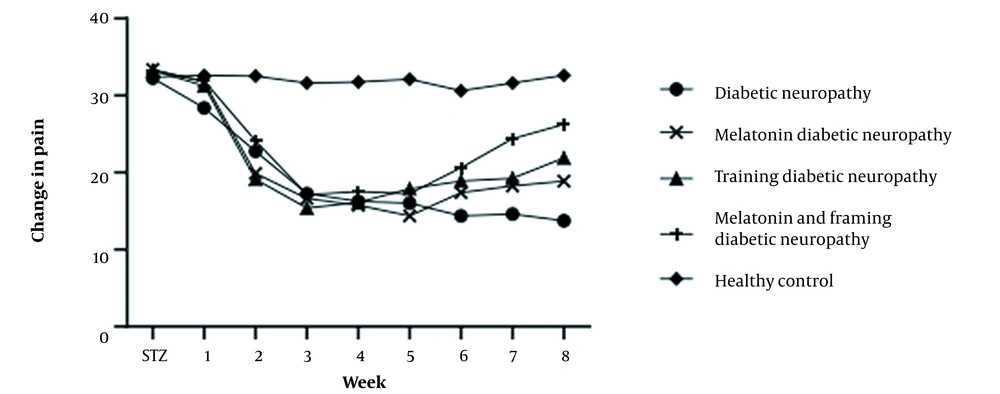
The findings showed no significant difference in the average paw withdrawal latency in the hot plate thermal hyperalgesia test (Figure 4) before streptozocin injection among the experimental groups (P < 0.05). The mean paw withdrawal delay was significantly lower in the diabetic groups than in the control group after two weeks of diabetes induction (P < 0.05).
4.1. TNF-α Expression
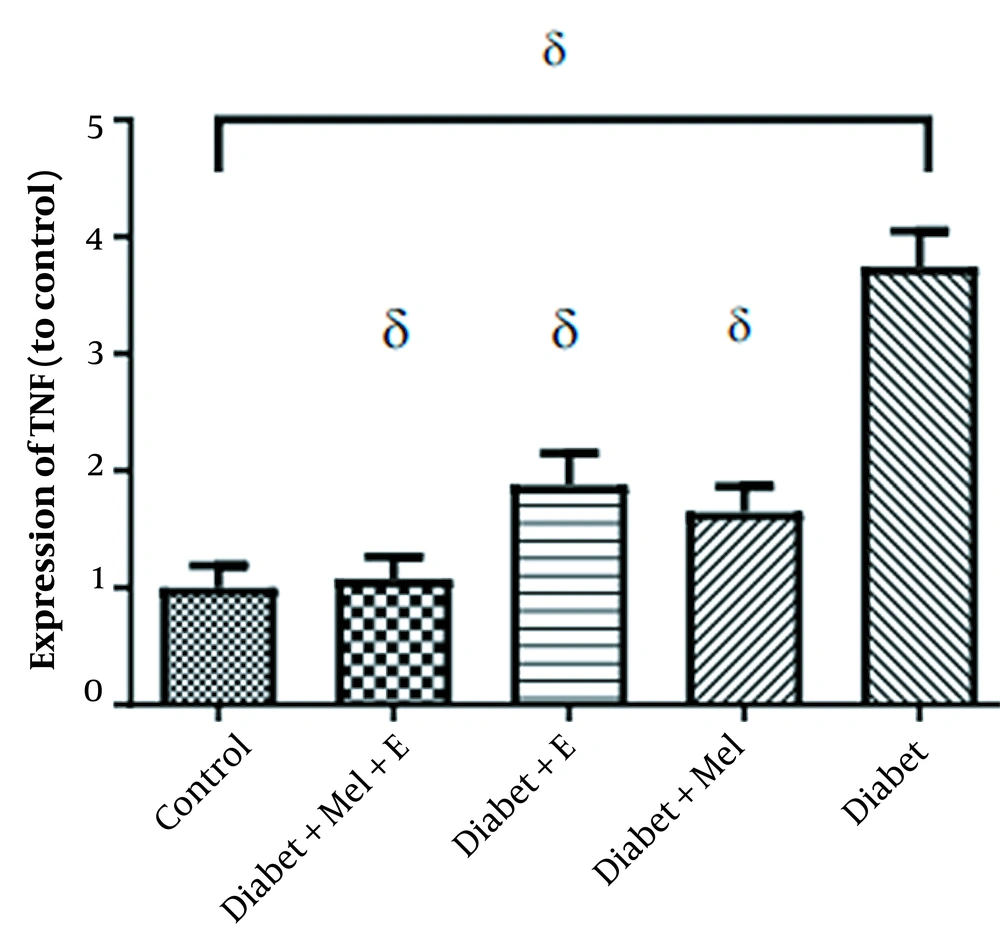
The mean ± SD of the TNF-α is shown in Table 2. The increase in TNF-α was significant when comparing the diabetic with the control group (t = 7.66, P = 0.0001). The induction of diabetes caused a significant increase in TNF-α gene expression. ANOVA analysis showed that there was a significant difference in TNF-α between the research groups (F = 21.84, P = 0.0001).
Also, the level of TNF-α gene expression was significantly lower in melatonin diabetic (P = 0.0001), training diabetic (P = 0.0001), melatonin diabetic (P = 0.0001), training diabetic (P = 0.0001), and control groups (P = 0.0001) than in the diabetic group (Figure 5).
5. Discussion
This research showed that two weeks after STZ injection, thermal hyperalgesia was seen in the diabetic group compared to the control group. Besides, when performing the hot plate test, the mean duration of paw withdrawal latency was significantly lower in the experimental diabetic groups than in the control groups, and this difference remained until the end of the test period. Also, after 6 weeks of aerobic training and melatonin injection, data from the hot plate examination revealed a significantly longer paw withdrawal in the training diabetic, melatonin diabetic, and melatonin and training diabetic groups than in the control group.
Studies have shown that inducing diabetes in rats by injecting STZ results in the entry of streptozotocin into pancreatic beta cells through the GLUT-2 glucose transporter. This causes DNA alkylation and reduces ATP levels, ultimately leading to the destruction of pancreatic beta cells. As a result, insulin secretion is reduced, and glucose homeostasis is disrupted (22). It has been proven that the induction of diabetes causes persistent hyperglycemia and hypoinsulinemia in animals, and therefore, the entry and accumulation of excess glucose in neurons triggers destructive metabolic pathways (23).
According to previous studies, the toxicity resulting from hyperglycemic conditions causes an increase in inflammatory mediators, such as cytokines like TNF-α, leading to an increase in pro-inflammatory cytokines, such as interleukin (9). The sensitivity of nerve receptors increases with the inflammatory activity caused by diabetes (10).
Research has shown increased neuropathic pain due to inflammatory responses (8, 24, 25). The findings of the present experiment confirmed previous studies. This research showed a significant increase in TNF-α expression in the sensory neurons of the posterior horn of the spinal cord in the experimental group compared to the control group. Inhibition of the signaling pathway is a solution researchers propose to reduce these conditions. One of the possible solutions to reduce the pain caused by diabetic neuropathy may be to inhibit TNF-α signaling (8, 26). Many studies have suggested that exercise may be a therapeutic method to reduce inflammatory factors (19, 24, 27); this research confirms this finding.
The impact of aerobic exercise on TNF-α expression reduction in nerve tissue of rats with diabetic neuropathic pain was studied. The results indicate that aerobic exercise positively affects sensory neurons and can reduce neuropathic pain. This effect is likely due to increased heat-shock proteins, anti-inflammatory cytokine levels, and glucocorticoids, which can inhibit the signaling pathway and reduce inflammation in peripheral nerves (28). Thakur et al. showed that aerobic training leads to the reduction of neuropathic pain associated with diabetes. In this case, the pain caused by streptozotocin in diabetic rats is relieved (24).
On the other hand, the current research investigated the effectiveness of melatonin supplementation as a non-invasive therapeutic intervention for diabetic neuropathic pain caused by inflammation at the cellular and receptor levels. The results showed that after six weeks of melatonin use in STZ-induced diabetic rats, there was a significant decrease in the expression of the pro-inflammatory cytokine TNF-α compared to the diabetic group. Numerous reports indicate the protective effects of melatonin against various complications of diabetes (14, 29, 30).
As mentioned, the amount of oxidative stress increases in diabetics (31). On the other hand, it has been shown that melatonin has an anti-oxidant role as an electron donor and can effectively improve oxidative damage in streptozocin-treated diabetic rats (32). Melatonin significantly inhibits high-glucose-induced ROS production, recovers mitochondrial membrane potential, and inhibits high-glucose-induced apoptosis in Schwann cells. In addition, melatonin reverses protein expression changes caused by high glucose treatment (30). Also, melatonin has been shown to reduce pain responses to various noxious stimuli and is considered a potential analgesic in the clinic (15).
Finally, this research showed that six weeks of aerobic training and melatonin consumption in STZ-induced diabetic rats significantly decreased the levels of TNF-α expression. Previous studies examined the interactive effect of melatonin consumption and combined training in various tissues. The results showed that this combination reduced the production of free radical byproducts, such as malondialdehyde, and effectively improved risk factors associated with diabetes in postmenopausal women (31).
5.1. Conclusions
The present study showed that in diabetic peripheral neuropathy, TNF-α expression increases and causes inflammatory conditions, increasing pain and sensitivity to pain in diabetic rats. Therefore, performing aerobic training with melatonin and the interaction of exercise and melatonin non-pharmacological strategies can moderate these changes and improve neuropathic pain caused by diabetes. However, no difference was observed between the effects of the mentioned interventions, and the effectiveness of the three methods was the same for the reduction of TNF-α.