1. Background
One of the most concerning diseases in the modern world is colon cancer. Colon cancer covers nearly 71% of colorectal cancer cases. As preventable and common as this disease is, in the last 2.5 decades, incidences of this disease have increased in people under 50 years of age. According to the world health organization (WHO), colon cancer ranks third most common in the United States (1-4). 1.8 million new cases of this disease are reported annually, and nearly 860000 patients lose their life because of colon cancer. 70% of reported cases are due to sporadic mutations, up to 25% are patients with familial history, and up to 10% are related to hereditary syndromes (1). Substantial intake of red meat and processed meat, extensive use of alcoholic drinks, inactive lifestyle, body fat, smoking, and various genetic factors are considered top risk factors by researchers and physicians (5, 6). The symptoms of this disease, abdominal pain, nausea and vomiting, constipation or diarrhea, and rectal bleeding are amongst the most common (5, 7). Currently, the preferred treatment method, along with surgery, is chemotherapy. Stage III and high-risk stage II patients usually undergo this treatment (8, 9). Oxaliplatin, 5-fluorouracil, capecitabine, and leucovorin are the most common pharmaceutical treatments in colon cancer chemotherapy. Chemotherapy can have harmful side effects influencing patients’ lives long after the treatment. These side effects can include vomiting, diarrhea, suppressed and weak immune system, and hair and weight loss (10-14). Therefore finding alternative treatment is of the highest importance.
2. Objectives
Linum usitatissimum (LU) is also known as Flax and belongs to the genus Linum and Linaceae family (15, 16). Flax is considered a beneficial dietary component that contains nutrients with anti-inflammatory, Oxidative, and antitumor properties such as alpha-linolenic acid (ALA omega-3), linoleic acid (LA), oleic acid, Lignan, vitamin B, and beta-carotene. It also contains many unsaturated fatty acids and fiber, as well as large amounts of potassium, magnesium, iron, copper, zinc, and various vitamins. It has been proven to have positive medicinal effects on some diseases, such as cardiovascular diseases and cancer (17-21). Considering these properties, we aimed to investigate the antiproliferative and apoptotic effects of L. usitatissimum seed aqueous extract on LS174T and COLO205 cancer cells and the expression of apoptotic genes BAX and BAD in comparison to normal colon cell line HCT116.
3. Methods
3.1. Aqueous Extract Preparation
Linum usitatissimum seeds were collected and dried in a dark place without moisture. After drying, they were completely crushed, and 300 grams of this grind was added to 1800 mL of distilled water. This mixture was heated at 40°C and stirred for 2 hours. This blend was set off for 24 hours at 25°C. Then the extract was smoothed by Whatman Filter Paper grade 2. The initial extract was evaporated in a vacuum distillation (rotary with a vacuum pump) at 80°C for 1 hour, and the final extract was obtained.
3.2. Cell Culture
Human colon cancer cell lines LS174t and COLO205 and normal colon cell line HCT116 were obtained from the Pasteur Institute of Iran and were cultivated in Roswell Park Memorial Institute (RPMI) 1640 medium enriched by adding 10% fetal bovine serum (FBS), 1% antibiotic blend of penicillin and streptomycin, 1% L-glutamine, and 1% non-essential amino acids. Flasks containing the cells were coated with gelatin to help the cells adhere better to the surface. Cells were incubated in a 37°C and 5% CO2 atmosphere.
3.3. Cell Viability (MTT) Assay
The cell viability assay (MTT) kit used in this study was purchased from (arsamsysbio.com). Each well of 96-well plates was filled with 3000 cells and an appropriate amount of medium before being incubated for a full day. The culture medium was changed the next day, and different doses of LU extract (2,4, 6, and 8 mg/ml) were added to the cells. The plates were then incubated for one and two full days, then 10% MTT was mixed in with the cells, and another 4 hours of incubation was commenced. The wells' contents were discarded afterward, a formazan solubilizing solution was mixed in instead, and optical density was rated at 560 nm.
3.4. mRNA Extraction
The manual method was used to extract RNA from the cell using the thiazol kit purchased from the ZiAViZ Company (ZiAzole). The process of mRNA extraction was performed according to the instructions given in the kit. The final blend containing the mRNAs was stored in a freezer.
3.5. Reverse Transcriptase PCR
Reverse Transcriptase (RT) PCR assay (Yekta Tajhiz kit) to convert mRNAs to cDNAs to investigate gene expression in real-time PCR. PCR master mix preparation and other steps were performed according to kits instructions. The final phase of RT-PCR was performed as follows: one hour of 42°C treatment followed by 5 minutes at 70°C.
3.6. Real-time PCR
The expression of BAX and BAD genes was assessed using Real-time PCR (qPCR). βactin was chosen to be the reference gene. The qPCR kits (Yekta Tajhiz) instructions were followed during the qPCR assay. 0.5, 1, and 2 mg/mL of LU extract were added to the cells for two full days before the assay. These doses were decided following the results obtained in the MTT assay. The sequence of the primers is as follows in Table 1.
| Gene | Forward | Reverse |
|---|---|---|
| Bata actin | 5'-GGAGTCCTGTGGCATCCACG-3' | 5'-CTAGAAGCATTTGCGGTGGA-3' |
| BAX | 5'- GGCCCACCAGCTCTGAGCAGA-3' | 5'- GCCACGTGGGCGGTCCCAAAGT -3' |
| BAD | 5'-CAGTGATCTGCTCCACATTC-3' | 5'-TCCAGCTAGGATGATAGGAC-3' |
The real-time PCR settings were as follows: (1) the first denaturation at 95°C 2 minutes; (2) each cycle was set to be 15 seconds of denaturation at 95°C, 15 seconds of annealing at 59°C, and 20 seconds of synthesizing at 72°C.
3.7. DATA Analysis
GraphPad Prism 9 (GraphPad Software, CA, USA) was used to analyze the data and prepare our graphs. P < 0.05 was chosen to be the statistical significance value.
4. Results
4.1. MTT Assay
MTT data after one and two days of treatment were analyzed.
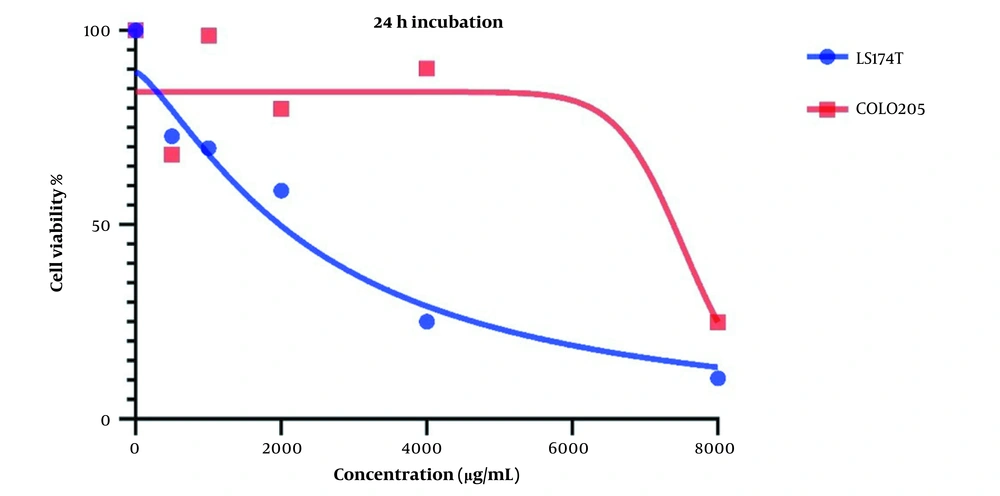
Changes in cell viability and the cytotoxic effect of LU extract on both cell lines after one day are presented in Figure 1. IC50 values for LS174T and COLO205 cells were 240 and 8719 µg/mL, respectively.
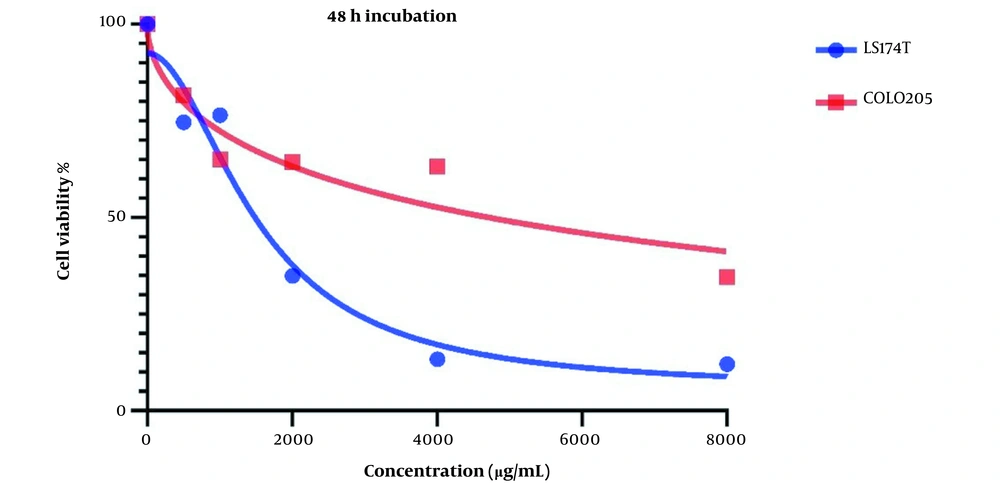
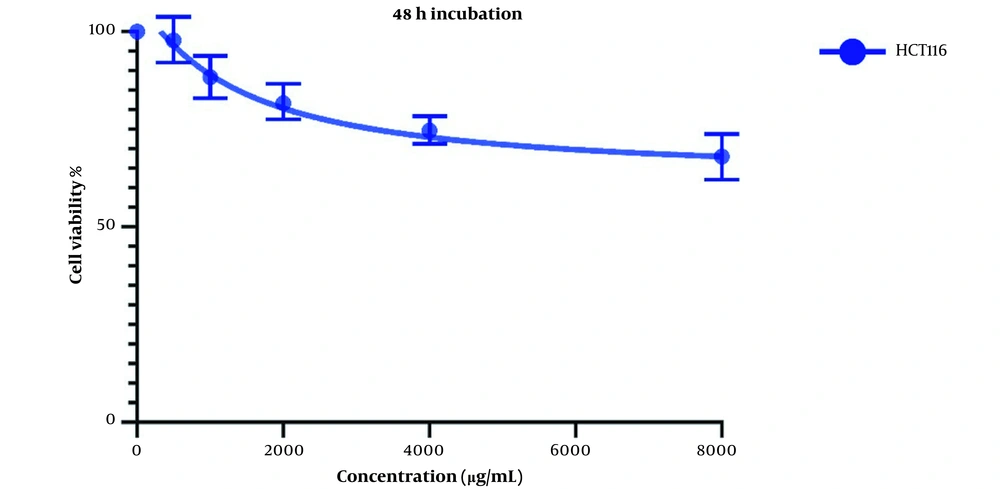
Figure 2 demonstrates Cell viability after two days of treatment with LU extract. IC50 values for these cells were calculated to be 1521 and 7832 µg/mL for LS174T and COLO205 cells.
Cell viability is affected in both cell lines after treatment with LU extract. This decrease in cell viability is directly related to the increase in LU extract concentration, which hints at a negative correlation between LU extract and its concentration and cell viability in cancer cells.
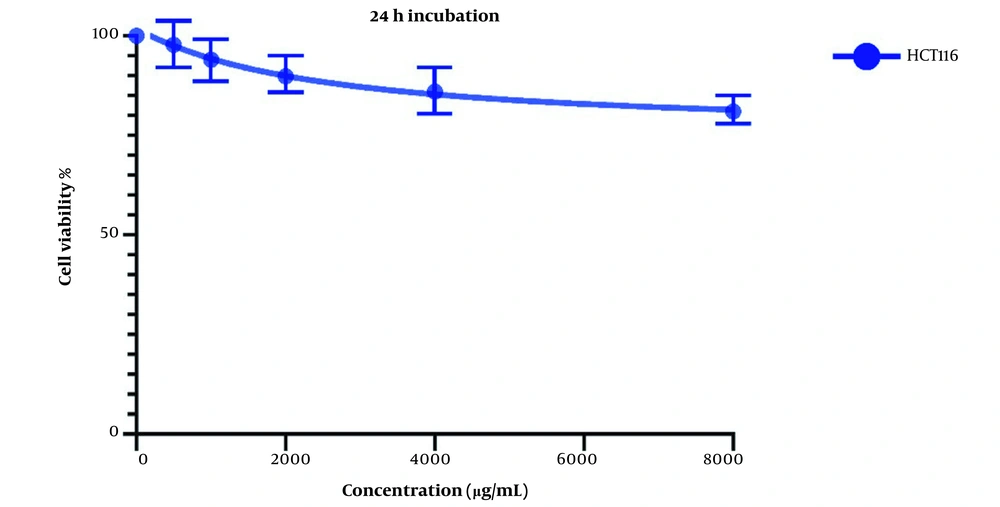
The same treatment in the same concentrations and in the same time frame was conducted on HTC116 normal colon cell line, and the IC50 values for one day of treatment (Figure 3) and two days of treatment (Figure 4) were calculated to be 2404 and 1389 µg/mL. Also, we observe a decrease in the cell viability of normal cells, and this decrease is statistically insignificant. These results indicate that LU extract only decreases the viability of cancer cells and is not harmful to normal cells.
4.2. qPCR
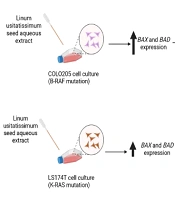
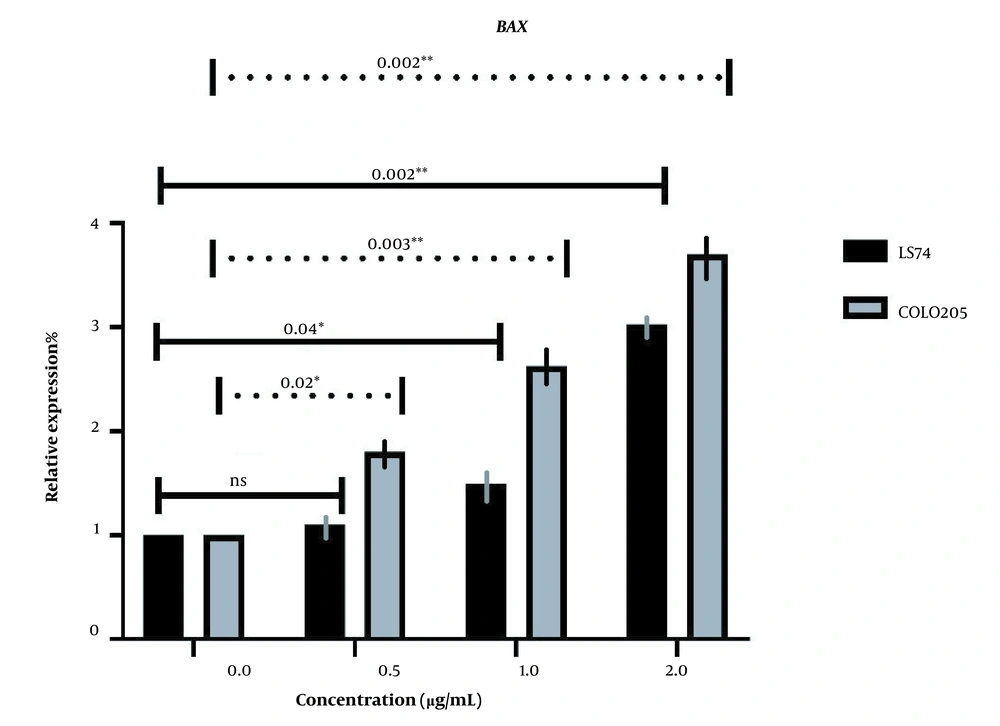
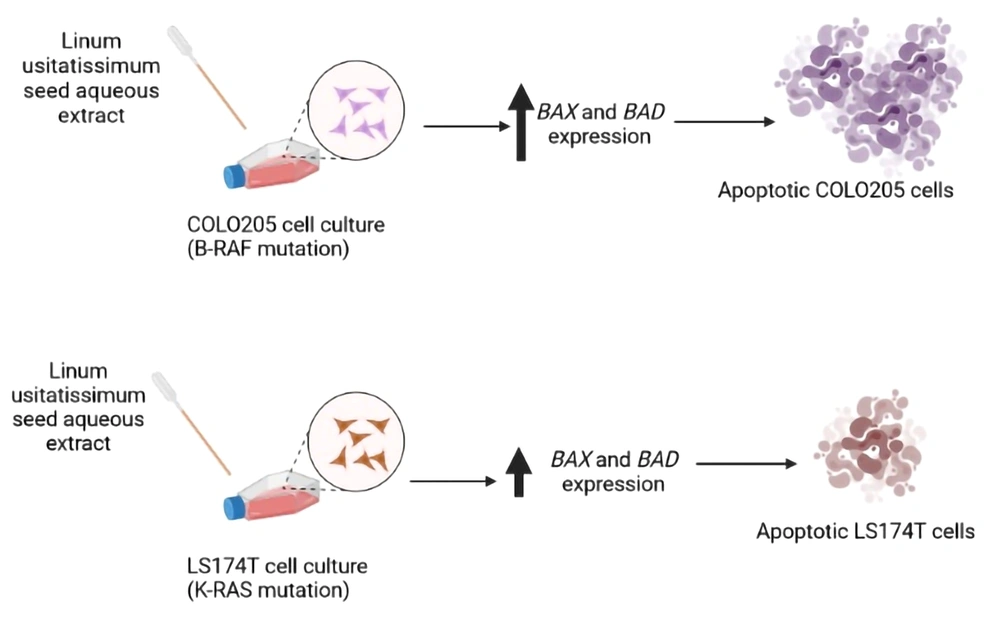
BAX and BAD expressions were investigated in both cell lines following the administration of different doses of LU extract to cells. The relative expression of pro-apoptotic BAX in both cell lines increased in correlation with increasing extract concentration (Figure 5). Almost all BAX expression changes were significant (P-value ≤ 0.05). The expression of BAX was increased more in COLO205 cells. Different mutations these cell harbor can cause this difference in sensitivity. LS174T cells contain K-RAS mutations, and COLO205 cells contain B-RAF mutations.
The relative expression of BAX in LS174T and COLO205 cells after treatment: The expression of BAX increased after treatment with different concentrations of Linum usitatissimum (LU) seed aqueous extract, and the increase in the concentration of OB extract was positively correlated with the increase of BAX expression.
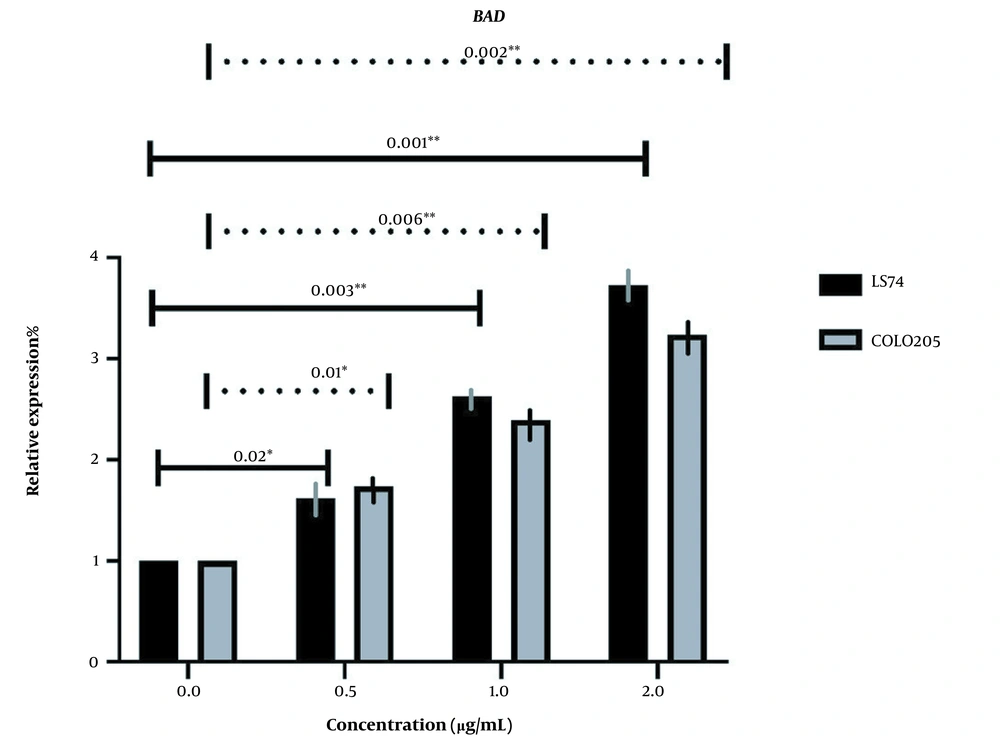
BAD expression was too changed in the same manner as BAX, increasing with the increase of extract concentration, and all the changes were significant (P-value ≤ 0.05) (Figure 6). However, BADs expression was increased more in LS174T cells which may also be associated with the different key mutations in our cells (Figure 7).
The relative expression of BAD in LS174T and COLO205 cells after treatment: The expression of BAD increased after treatment with different concentrations of Linum usitatissimum (LU) seed aqueous extract, and the increase in the concentration of OB extract was positively correlated with the increase of BAD expression.
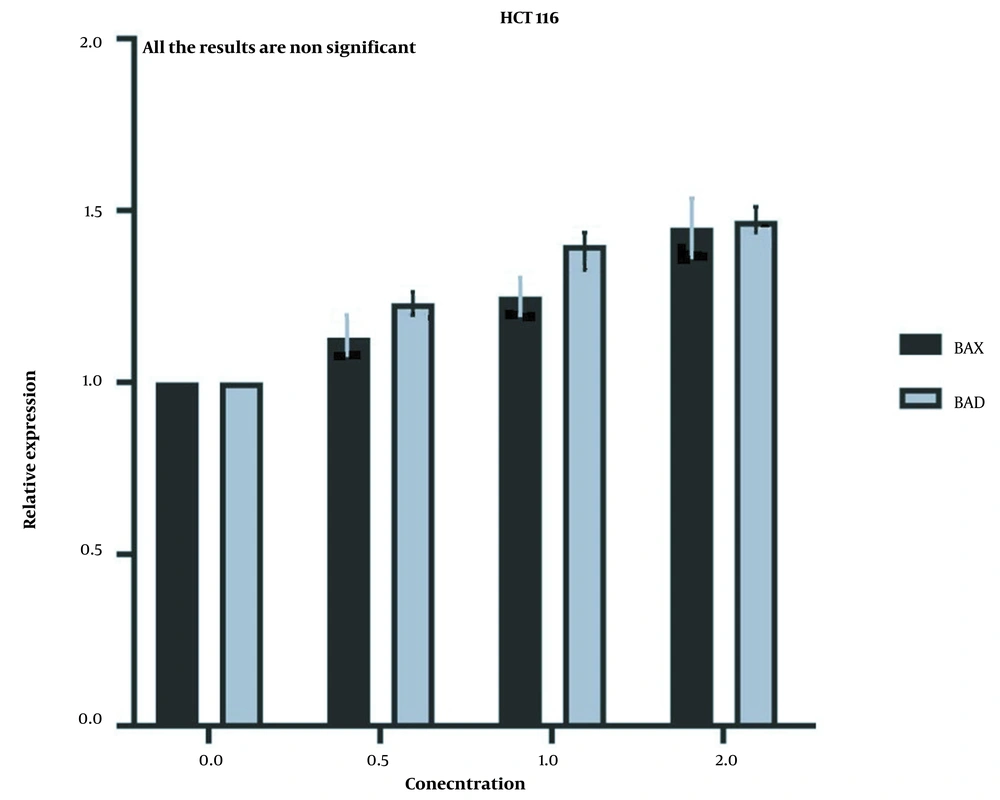
But the expression of the aforementioned genes did not show a significant increase in HCT116 normal cells (Figure 8) after the same treatment was applied to them, which further proves that the LU extract is not harmful to normal cells.
5. Discussion
The colon cancer epidemic has plagued the modern world for years. Chemotherapy is often the preferred treatment method for treating this disease. Nevertheless, chemotherapy and the drugs used during treatment can have harmful side effects from which patients can suffer even after treatment is complete. This inquiry aimed to research the anti-cancer effects of L. usitatissimum seed aqueous extract on colon cancer cells compared to normal colon cell lines to try and provide an alternative route of approaching colon cancer treatment.
We utilized MTT assay and qPCR to investigate our hypothesis. MTT assay data concluded that our extract has cytotoxic effects on both cell lines at 24 and 48-hour marks. The best results were achieved after 48 hours of treatment, and as a whole, LS174T cells were affected better at both time marks. This means that our extract induced apoptosis in both cell lines, but its ability to induce apoptosis in COLO205 cells was higher. Different mutations in the COLO205 cell and LS174T cell may cause this difference, but this theory needs further investigation. Our investigation also revealed that no significant changes in cell viability were observed after the same treatment was applied to normal HCT116 cells, which means that this extract is not harmful to normal cells.
The expression of apoptotic genes was investigated as well. The expression of pro-apoptotic BAX and BAD was increased after treatment with LU extract in correlation with increasing extract concentration used to treat the cells. BAX was increased more in COLO205 cells than BAD, which was increased more in LS174t cells. This difference in the relative expression changes can also be associated with different mutations in cell lines, but further investigations are needed in this area as well. The expression of these genes showed no significant changes in normal cells after the same treatment, which further shows that our extract does not harm normal non-cancerous cells.
Considering all the results, we can conclude that LU extract can contain various components mentioned before that can have antiproliferative and pro-apoptotic qualities and properties. Future investigations can look deeper into this area to determine which components are responsible for these results.
In a study by Ajay Bommareddy et al., the effects of dietary Flax were investigated on APCmin mice. The results revealed that the number of tumors in the small intestine and colon and their size significantly decreased in the flax-treated group compared with the corn-treated and the control groups. The presence of Ω3 fatty acids and lignan was increased in the serum, intestine, and colon of the flax-treated group, while the expression of COX1 and two was decreased in this group (22).
A 2010 study concluded that Secoisolariciresinol diglucoside rich extract (SRE) of L. usissatisimum (flaxseed) could prevent type 2 diabetes Mellitus related colon cancer by inhibiting cyclin-dependent kinase 4 (CDK4). This study treated sick mice with 500 mg/kg of SRE for up to 24 weeks, and the results showed that in addition to decreasing CDK4, hyperglycemia, hyperinsulinemia, pro-inflammatory cytokines, cancer biomarkers, and the number of proliferating cells decreased considerably (17).
Based on previous studies on the effects of dietary flaxseed on APCmin mice, Bommareddy et al. conducted an experiment to determine the effects of flaxseed components lignan (enterodiol and enterolactone), Ω3 fatty acid α-linolenic acid on human colon adenocarcinoma cells (Caco-2 cells). The results of cell viability and flow cytometry assay concluded that these components caused a significant increase in apoptotic cells and a significant decrease in cellular proliferation (23).
Another study investigated the effects of lignan enterolactone (ENL), found in flaxseed, on COLO201 cells. MTS assay results suggested that this component has cytotoxic effects on COLO201 cells. Western blotting revealed that the expression of BCL2 is downregulated, and the amount of cleaved caspase three is upregulated significantly. This study also reported that the expression of p53, BAX, BCL-xl, BCL-s, and caspase eight was not affected by this component. After treatment, these cells were transported to athymic mice and showed no ill side effects. These results suggest that this component of flaxseed has pro-apoptotic properties (24).
5.1. Conclusions
Considering our results and previous results mentioned above, we can assume that the components found in L. usitatissimum seed aqueous extract have antiproliferative and pro-apoptotic properties and can halt cancer growth and promote apoptosis while not harming normal cells in the process. Future inquiries are needed to study better the effects of components found in LU extract to determine their effects on cancer cells. Future studies should also consider a more in-depth look at the effects of this extract and its components on different mutations of colon cancer to help provide a better future for treating colon cancer.