1. Background
Suturing is the final step in most oral surgery procedures. After surgery, the sutures serve important functions as they facilitate joining the separated tissues again in addition to promoting primary healing and control of the bleeding (1). The sutures must not, either directly or indirectly, cause inflammation. In order to reduce the inflammatory process and promote wound healing, it is crucial to minimize plaque retention and, thereby the presence of microorganisms. In this sense, it is necessary to limit the adhesion and proliferation of bacteria in the tissues contacting oral fluids in oral surgery (1). Thus, materials with low retention of bacteria should be used in the frame of protocols that minimize the effects of poor cleaning on healing processes (2).
Since sutures used after oral surgery procedures (e.g., extraction of unerupted teeth) promote adherence of pathogenic bacteria, they are considered a risk factor for the healing of surgical wounds.
The species of genera Fusobacterium, Peptostreptococcus, Prevotella, Porphyromonas, Streptococcus, and Bacteroides have been reported to belong to the accumulated bacteria family. Different species of Candida could be part of the dental plaque and thus be carried just as the accumulated bacteria are carried.
The presence of bacteria in blood and odontogenic infections have been associated with suture removal and a potential risk for bacterial endocarditis (3). Candida has also been associated with endocarditis (4). To date, however, no study has investigated the colonization by fungi.
A high increase of fungal infections has been reported over the last decades. It includes yeasts, with Candida spp. as the absolute dominating pathogen. Such infections, called candidiasis, can develop in open wounds as complications of diverse types of surgery. For example, C. albicans has been detected after cardiac surgery in skin biopsy and cultures, which causes local and distant infections such as severe mediastinitis (5).
Since the yeasts are considered part of the normal human micro-biota, invasive infections only occur in immunocompromised patients or when barrier leakage is impaired. For example, yeasts can enter the bloodstream and cause fungaemia and subsequent infections. Candida is the most common fungal pathogen that produces fungaemia.
Tight adherence to human cells from skin, epithelium or endothelium is the first step in Candida infections. The efficacy to bind those host tissues and/or fomites (e.g., catheters or prosthetic devices) depends on adhesins which are substances detected in fungal cell walls and codified by genes (6).
The ability of Candida species to colonize host tissues and cause disease is influenced by diverse virulence factors such as biofilms development. A biofilm is a particular community of cells that can grow either in abiotic or biotic substrates, including mucosal surfaces and suture materials (7).
Candida albicans biofilms have been associated with persistent high virulence factors, drug resistance, and death increases. In biofilms, C. albicans can bear synergistic interactions with Streptococcus mutans, thus promoting bacterial colonization as well as developing caries and risk of other diseases (7).
Different species of Candida, playing a commensal or opportunist role, are very important factors involved in the adherence of bacteria to soft tissues and further in the deep invasion. In fact, their hyphae have been seen invading the connective tissue in association with anaerobic microorganisms (Porphyromonas gingivalis, Prevotella intermedia, and Aggregatibacter actinomycetemcomitans).
Adherence and tissue penetration are facilitated by extracellular hydrolytic enzymes, with the subsequent Candida overgrowth (8, 9).
According to their decreasing virulence, Candida species are classified into three groups, namely (1) C. albicans and C. tropicalis; (2) C. glabrata, C. lusitaniae, and C. kefyr; and (3) C. krusei, C. parapsilosis and C. guilliermondii (10).
Sutures are believed to retain Candida spp. From this site, it could spread to different areas and even cause sepsis, especially in immunosuppressed patients.
2. Objectives
The present study aimed to evaluate the presence of Candida species in threads after the extraction of retained third molars as well as to examine the influence of the position of dental piece and the used material.
3. Methods
A total of 56 male and female patients aged between 21 - 55 years and with retained lower third molars in a similar position and angulation on both sides were examined. The patients participating in this study were immunocompetent and non-smokers and had received no antimicrobial treatment one month before taking the sample. All patients signed an informed consent approved by the Ethics Commission of the Faculty of Dentistry of the University of Buenos Aires. Panoramic X-ray images were taken to diagnose the third molar position. The teeth extraction was performed according to routine technique, and the gum tissues were sutured using random nylon and silk threads.
In the next session, a week after the third molar extraction, the sutures were removed using sterile scissors and making a cut under the triple knot of silk thread and, then, were placed in an Eppendorff tube. The same microbiological techniques were implemented, and fresh microscopic studies, as well as Giemsa and Gram stains, were carried out. The yeasts were grown in differential chromogenic medium (CHROMagar Candida®BD, Paris, France) and in Sabouraud Dextrose Agar (SDA; Becton Dickinson) and were incubated at 37°C for one week. Then, the different species were identified based on the color they developed in the chromogenic medium. Thus, the presence of one or more species were detectable in the assays. The micromorphology was studied on 1% milk-Tween 80 agar, and a carbohydrate assimilation test was carried out using the commercial API® ID 32D system and Vitek2. In addition, the species developing green color in the chromogenic medium were evaluated by a xylose assimilation test, and growth at 45°C. Isolates with presumptive identification of C. dubliniensis underwent DNA extraction to later amplify with species-specific primers.
Statistical analysis was performed using Statistix 7.0 and SPSS 11.0 versions. Confidence intervals (CI) were calculated at 95% employing the Epi-Info 6.04 program (Atlanta University, USA).
4. Results
4.1. Prevalence of Yeasts and Their Species in Suture Threads
Out of 56 patients examined in this study, 16 ones (28.57%) were found to carry some species of yeast of the genus Candida (Table 1). The predominant species was C. albicans (13 patients; 23.20%), followed by C. parapsilosis complex (3 patients; 5.40%). These percentage values were calculated with respect to the total number of patients (56). The percentage values of the total yeast-positive patients (16) were 81.25 and 18.75 for C. albicans and C. parapsilosis complex, respectively. The confidence interval of 95% (CI 95%) was 13.4 - 36.7 for C. albicans, and it was 1.1 - 14.9 for C. parapsilosis (Table 1).
| Candida Species Carried | Yeast-Positive Patients | |
|---|---|---|
| No. (%) | CI 95% | |
| C. albicans | 13 (23.20) | 13.4 - 36.7 |
| C. parapsilosis complex | 3 (5.40) | 1.1 - 14.9 |
| Any species | 16 (28.57) | |
a The total number of patients (n) was 56, and 16 patients were yeast-positive. The percentage value consigned here was calculated in relation to the total number of patients. The confidence interval (95%) was given. Materials from patients were analyzed according to materials and methods.
4.2. Influence of the Third Molar Position
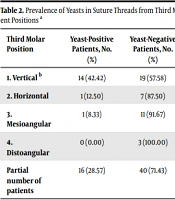
Yeasts were detected in the vertical position of the third molar in 14 out of 33 patients (42.42%), although 19 of them tested negative (57.58%) (Table 2). Horizontal and mesiangular positions were less associated with yeasts (1/8; 12.50% and 1/12; 8.33%, respectively; *P (Fisher) = 0.8 (CHI2 Yates) = 0.6027; GL = 1; P = 0.4376). No statistically significant difference was observed among the positions.
| Third Molar Position | Yeast-Positive Patients, No. (%) | Yeast-Negative Patients, No. (%) | Total Patients, No. (%) |
|---|---|---|---|
| 1. Vertical b | 14 (42.42) | 19 (57.58) | 33 (100.00) |
| 2. Horizontal | 1 (12.50) | 7 (87.50) | 8 (100.00) |
| 3. Mesioangular | 1 (8.33) | 11 (91.67) | 12 (100.00) |
| 4. Distoangular | 0 (0.00) | 3 (100.00) | 3 (100.00) |
| Partial number of patients | 16 (28.57) | 40 (71.43) | 56 (100.00) |
a Materials from 56 patients were analyzed according to materials and methods.
b Statistical analyses: *P (Fisher) = 0,8 (CHI2 Yates) = 0,6027; GL = 1; P = 0,4376).
4.3. Influence of the Suture Thread Material
Regarding the material used in the suture, yeasts were detected in 9 out of 26 (34.61%) patients with nylon threads and in 7 out of 30 (23.33%) patients with silk threads (Table 3).
| Suture Material | Positive Threads, No. (%) | Negative Threads, No. (%) | Total Threads, No. (%) |
|---|---|---|---|
| Nylon | 9 (34.61) | 17 (65.38) | 26 (100.00) |
| Silk | 7 (23.33) | 23 (76.67) | 30 (100.00) |
| Total | 16 (28.57) | 40 (71.43) | 56 (100.00) |
a Materials from 56 patients were analyzed according to materials and methods. P (Fisher) = 0.262367; CHI2 (Yates) = 0.4038; GL = 1; P = 0.5251.
Although a lower risk trend was inferred for silk, the statistical analysis revealed no significant differences between the two materials.
5. Discussion
Saliva, great vascularization, and tissues playing roles in swallowing, mastication, and speech make the sutures used in oral and maxillofacial surgery different from those used for other parts of the body (1).
Regarding oral surgery, the extraction of third molars requires careful consideration due to well-documented disorders associated with them, which are different from other teeth (11). The quality of healing after the operation depends on the effectiveness of the sutures. In this context, they should limit the adhesion and proliferation of bacteria to those parts that contact oral fluids, thus avoiding wound contamination (1, 12).
The adhesion of aerobic and anaerobic bacteria to the suture threads was already demonstrated (1), although the presence of fungi was not reported. Our study was the first report on the presence of fungi in post-extraction suture threads of retained third molars.
According to our study results, C. albicans was present on the threads suture (Tables 1 - 3). This result was consistent with the findings from previous studies reporting the adherence of C. albicans to different materials as well as to epithelial cells. Our results also showed that C. albicans was present with a higher proportion than C. parapsilosis (23.20% and 5.40, respectively). Unlike C. albicans, little is known about the adhesion of C. parapsilosis and its role in recognizing the host cell surface (13-15).
Lima-Neto et al. (16) examined the correlation of the adherence of C. albicans and C. parapsilosis strains with human buccal epithelial cells, and showed that C. albicans had much higher adherence than C. parapsilosis.
The C. parapsilosis complex stands out for causing nosocomial infections worldwide and behaves as an opportunistic fungal pathogen. It is a complex of three species, namely C. parapsilosis sensu stricto, C. orthopsilosis, and C. metapsilosis, which are different from a genetical point of view (17).
The transition from yeast to pseudohypha growth promotes C. parapsilosis invasion through several mechanisms, including disruption of host cells and tissues, penetration of tissues, and formation of biofilms (18, 19).
C. parapsilosis sensu stricto has been commonly associated with colonization of the oral mucosa, particularly in pathological conditions. This colonization can turn the mouth into a potential source of either candidemia, invasive mycoses, or direct person-to-person contact fungal transmission (20, 21).
Various factors could affect the prevalence of yeasts in sutures, out of which the factors, including the position and angulations of a dental piece as well as the material used in the sutures were investigated in our study. Our results demonstrated that the vertical position of the molar was likely to carry yeasts to a greater degree than the horizontal and mesiangular one; however, it was not statistically significant (Table 2).
There is extensive literature on suture materials. Research has led to developing different types of suture materials, including commercially available natural and synthetic, absorbable, and non-absorbable ones (1). Traditionally, cotton and silk sutures are the most common materials used for skin closure. Studies have shown that nylon is usually the first selection since it has lower bacterial retention than silk, polyester, and Vyeril (22).
In retained third molars surgery, silk has shown a higher percentage of bacterial retention, with greater content of cocos and anaerobic bacilli that are more aggressive (2). By contrast, our results showed that silk tended to retain yeasts of 23.33%, which was lower than that of nylon (34.61%), although the difference was not statistically significant. This value may have changed in further studies with larger population (Table 3).
Our results confirmed the inclusion of antifungal agents in suture materials. Baygar et al. (23) used silk and coated the sutures with biologically synthesized silver nanoparticles. These non-absorbable materials had in vitro antimicrobial effects on the bacteria Staphylococcus aureus and Escherichia coli, in addition to fungi such as C. albicans. Overall, our results may have provided relevant insights into the prevention of the microbial accumulation and optimization of patient management.