1. Background
Free radicals, especially reactive oxygen and nitrogen species (RONS), play a critical role in the pathogenesis of various diseases such as cancer, diabetes, hypertension, cataract, arthritis, atherosclerosis, and cardiovascular and respiratory disorders (1), as antioxidants trap these species (2). Oxidative stress occurs when a living organism has a low concentration of antioxidants (3). Compounds in herbal extracts, including flavonoids, alkaloids, terpenes, glucosinolates, tocopherols, carbohydrates, retinoids, carotenoids, thiols, polyphenols, and trace metals, can control the concentrations of RONS (4). Scientists have focused on phytochemicals to find novel effective, harmless, and inexpensive antimicrobial agents. The increasing emergence of antibiotic-resistant bacteria and fungi with grave consequences are of great concern (5). Microbial resistance to antibiotics and global warming is intertwined, and some researchers believe that antibiotic resistance’s costs and harmful effects are more than that of climate change (6-8).
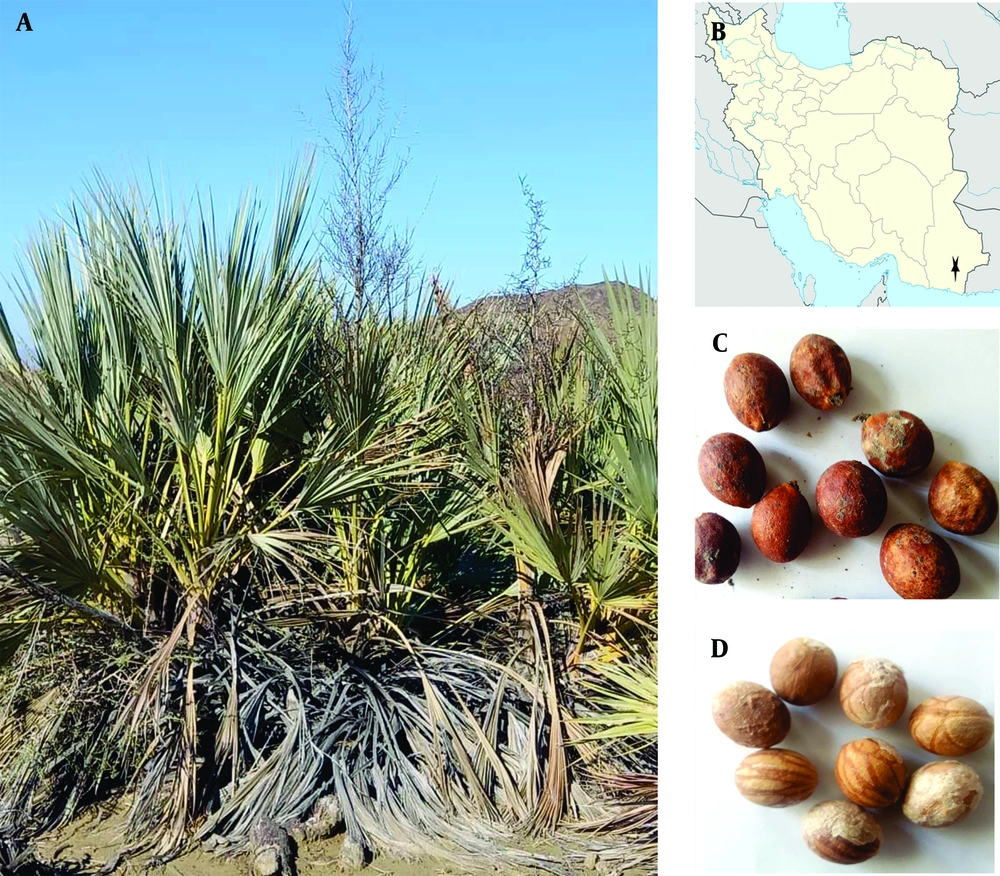
Nannorrhops is a shrubby palm genus in the family Arecaceae (Palmae), encompassing two species in the Saharo-Sindian region. Nannorrhops baluchestanica Khodash, locally called Daz or PORK, is a recently discovered species from Iranshahr in SE Iran (9, 10). However, the species distribution range seems to be much wider than the reported locality. Nannorrhops is consisted from two Greek words (nannos + rhops), meaning small shrub, and baluchestanica refers to the geographical region where the plant was first identified in 2016. This species is an attractive shrub closely related to N. ritchiana but differs from it through a series of morphological characteristics, including bigger stems, floccose hairs resulting in silvery leaves, rather distinct and globose carpels, large, bitter, and light brown fruits and late flowering time. The plant is readily distinguished by native people and is widely used, along with N. ritchiana, for various purposes, such as making a variety of handicrafts and relieving constipation. Due to limited distribution range and small population size, endemic species are vulnerable to extinction at a much higher rate compared to other species. On the other hand, these valuable gene pools, which are only accessible to local scientists, can be of potential industrial, medicinal, or economic use. To the best of our knowledge, there is no scientific data on the biological properties of N. baluchestanica in the literature.
2. Objectives
In this project, the antioxidant and antimicrobial capacities of the hydroethanolic extracts of seeds and fruits of N. baluchestanica were analyzed as the first step towards recognition of their potential as antibiotics or treatment of oxidative stress-related and infectious diseases.
3. Methods
3.1. Plant Sampling and Extract Preparation
The seeds and fruits of N. baluchestanica were collected in autumn 2022 from Shark village (Alt/Lat 26.0245, 61.0691), located in SE Ghasreghand, Sistan and Baluchestan, Iran. The third author identified the plant species as N. baluchestanica Khodash. A herbarium sample was preserved in the University of Zabol Herbarium (under voucher code: UOZH 1504). Figure 1 shows the species’ habitat, habits, and details of the fruits and seeds’ morphology. The seed and fruit hydroalcoholic extract preparation followed our previous works on N.ritchiana and Stocksia brahuica, where the berries and seeds were separated, airdried away from sunlight, milled into powders using a moulinex masterchef blender, and stored at 5°C in the refrigerator (11, Author’s Unpublished Work). To extract the biologically active ingredients of the seeds and fruits, 10 g of each powder was separately macerated in 100 mL of the hydroalcoholic solvent (50% water + 50% ethanol) on an electronic shaker for 72 hours. Then the solutions were filtered through a Whatman paper, and the filtrate was left to evaporate at 37°C. Finally, the dried mass was dissolved in DMSO 10% to obtain desired concentrations (11, Author’s Unpublished Work).
3.2. Antioxidant and Antimicrobial Activity
α-Tocopherol (vitamin E), 2,2-diphenyl-1-picrylhydrazyl (DPPH), fluconazole, gentamicin, and ampicillin were purchased from Sigma-Aldrich. Fungal strains, including Aspergillus fumigatus (Persian type culture collection (PTCC) 5009), Fusarium oxysporum (PTCC 5115, CBS 620.87), and Candida albicans (PTCC 5027, ATCC 10231), Gram-negative bacterial strains including Klebsiella pneumoniae (PTCC 1290, NCTC 5056), Pseudomonas aeruginosa (PTCC 1310. ATCC 10145), and Escherichia coli (PTCC 1399, ATCC 25922), and Gram-positive bacterial strains including Bacillus cereus (PTCC 1665, ATCC 14579), Staphylococcus epidermidis (PTCC 1435, ATCC 14990,) and Streptococcus pyogenes (PTCC 1447, ATCC 12204) were procured from the PTCC, Tehran, Iran. To determine antioxidant and antimicrobial properties, DPPH free radical scavenging protocol and broth microdilution and streak plate methods were applied (12, 13). The absorbance of microbial suspensions and methanolic solutions containing DPPH was measured using a UV-2100 RAY Leigh UV-VIS spectrophotometer. All biological results were expressed as the average of three independent experiments.
3.3. IC50 Determination
Methanolic solutions at the concentrations of 12.5, 25, 50, and 100 µg.mL-1 were prepared for both extracts, and 1 mL of each was mixed with 3 mL of DPPH methanolic solution (0.004%). The resulting solutions were kept at room temperature in darkness for 30 min. Then, the absorbance was recorded at 517 nm against a blank. The inhibition percentage (I%) was calculated according to the following equation:
A straight-line graph of I% vs. concentration was drawn, and its equation was determined as:
"x" is the half maximal inhibitory concentration (IC50) while y = 50.
3.4. Preparation of Microbial Culture Media
Mueller-Hinton broth (MHB), sabouraud dextrose broth (SDB), Mueller-Hinton agar (MHA) and sabouraud dextrose agar (SDA) was prepared by dissolving 21, 30, 38, and 65 g of the corresponding medium in 1000 mL distilled water, respectively. The solutions were sterilized by autoclaving at 121°C for 15 minutes.
3.5. Preparation of Microbial Suspensions
0.5 McFarland standard of all microorganisms were prepared in the corresponding broth media. For this purpose, the absorbance of bacterial and fungal suspensions at 625 and 530 nm wavelengths were set to be 0.08 - 0.1 and 0.12 - 0.15, respectively. Finally, bacterial and fungal suspensions were diluted 300 and 100-fold, respectively.
3.6. MIC Determination
An initial solution of each extract with a concentration of 4096 μg.mL-1 was prepared in dimethyl sulfoxide (DMSO). Twenty µL of the solution was added to the first and second wells in a row of a 96-well microliter plate. Twenty µL DMSO was added to wells 2 - 12, and two-fold serial dilutions were carried out. Eighty µL of MHB or SDB and 100 µL of diluted microbial suspensions were added to all wells to achieve an extract concentration range of 4096 - 2 μg.mL-1. The plates were incubated with shaking at 100 rpm at 37°C for 24 h. The minimum inhibitory concentration (MIC) was identified as the lowest extract concentration without visible turbidity.
3.7. MBC and MFC Determination
Samples of all wells without visible turbidity in the MIC test were cultured in MHA or SDA media plates. Plates were incubated at 37°C for another 24 h. The minimum bactericidal concentration (MBC) or the minimum fungicidal concentration (MFC) was determined as the lowest concentration of extracts in which no microorganisms survived.
3.8. Statistical Analysis
The mean differences were calculated using Duncan’s multiple range tests with a 95% confidence interval (P ≤ 0.05).
4. Results
4.1. Antioxidant Effects
2,2-diphenyl-1-picrylhydrazyl is a relatively stable free radical that possesses an intense violet color. It is scavenged by antioxidants, and the color changes to yellow. Indeed, antioxidants reduce it via hydrogen atoms and/or electron donating. 2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity of N. baluchestanica extracts was investigated to assess its antioxidant potential (Table 1).
| Variable | Extracts | Drug | |
|---|---|---|---|
| Fruit | Seed | α-Tocopherol | |
| IC50a (µg.mL-1) | 26.20 | 33.94 | 10.40 |
Antioxidant Activity of the Hydroalcoholic Extracts of the Seeds and Fruits of Nannorrhops baluchestanica and the Positive Control
Considerable antioxidant effects were observed with extracts compared to vitamin E. The fruit extract was significantly more effective in scavenging DPPH than the seed extract (P = 0.05).
4.2. Antibacterial and Antifungal Effects
The excellent antioxidant activities of N. baluchestanica encouraged us to study its antimicrobial potentials. For this purpose, both extracts’ in-vitro blocking properties were compared to standard drugs (Table 2).
| Bacterial and Fungal Strains | Extracts | Drugs | |||
|---|---|---|---|---|---|
| Fruit | Seed | Gentamicin | Ampicillin | Fluconazole | |
| Escherichia coli 1399 a | |||||
| MIC | 256 | ND | 8 | 32 | - |
| MBC | 256 | ND | 8 | 64 | - |
| Pseudomonas aeruginosa 1310 | |||||
| MIC | 128 | 1024 | 0.063 | 1024 | - |
| MBC | 128 | 1024 | 0.063 | 2048 | - |
| klebsiella pneumoniae 1290 | |||||
| MIC | 2048 | ND | 4 | 32 | - |
| MBC | 2048 | ND | 4 | 64 | - |
| Bacillus cereus 1665 | |||||
| MIC | ND | ND | 0.25 | 32 | - |
| MBC | ND | ND | 4 | 64 | - |
| Streptococcus pyogenes 1447 | |||||
| MIC | 1024 | ND | 2 | 4 | - |
| MBC | 1024 | ND | 2 | 8 | - |
| Staphylococcus epidermidis 1435 | |||||
| MIC | ND | ND | 1 | 0.25 | - |
| MBC | ND | ND | 2 | 2 | - |
| Candida albicans 5027 | |||||
| MIC | 1024 | ND | - | - | 256 |
| MFC a | 2048 | ND | - | - | 512 |
| Aspergillus fumigatus 5009 | |||||
| MIC | 2048 | ND | - | - | 32 |
| MFC | 2048 | ND | - | - | 64 |
| Fusarium oxysporum 5115 | |||||
| MIC | 1024 | 256 | - | - | 128 |
| MFC | 1024 | 256 | - | - | 256 |
The Inhibitory, Bactericidal, and Fungicidal Effects of Nannorrhops baluchestanica Extracts and Drugs
For the bacterial strains, the MIC and MBC values of both extracts ranged from 128 (P. aeruginosa) to 2048 µg.mL-1 (K. pneumoniae). Concerning the fungal strains, the MIC and MFC values ranged from 256 (F. oxysporum) to 2048 µg.mL-1 (A. fumigatus and C. albicans).
As shown in Table 2, the hydroalcoholic fruit extract inhibited the growth of all microbial strains except for B. cereus and S. epidermidis. However, P. aeruginosa and F. oxysporum were the only strains inhibited by the seed extract. The fruit extract seemed more effective in inhibiting the growth of Gram-negative strains than Gram-positive strains. The most sensitive pathogens to the fruit and seed extracts were P. aeruginosa and F. oxysporum, respectively. However, the MIC, MBC, and MFC for plant extracts were considerably higher than those of gentamicin, ampicillin, and fluconazole, revealing the weak efficacy of seed and fruit extracts of N. baluchestanica against the tested pathogens.
5. Discussion
Plant-based bioactive compounds with antioxidant and antimicrobial activity have increasingly become a focus of scientific research. This research revealed remarkable antioxidant capacity and modest antimicrobial activity of the fruit and seed extracts of N. baluchestanica. There is no previously reported antioxidant activity for N. baluchestanica, but there are some sparse works on the antioxidant properties of different organs of its taxonomically close species N. ritchiana. Inhibitory activities of hydromethanolic extract of N. ritchiana Griff. leaves and their fractions were evaluated against DPPH, where the inhibition percentage values at a concentration of 10 μg.mL-1 ranged from 14.73 to 75.88 (14).
Evaluating the ability of the alcoholic extracts of the seeds and fruits of N. ritchiana to reduce the stable radical DPPH to the yellow-colored DPPH-H revealed that the IC50 value for seed extract was lower than that of the fruits (117 vs. 204 μg.mL-1 respectively). This indicates that the seeds have a stronger ability to react with oxidants than the fruits of N. ritchiana (Author’s Unpublished Work). This contradicts the results of this study on seeds and fruits of N.baluchestanica, showing the fruit extract was significantly more potent than the seed extract in quenching the DPPH radicals. This contradiction may be related to genetic differences between the two species. This deviation may also be caused by the difference in laboratory conditions, such as the storage time of the seeds and fruits and the corresponding powders since, in contrast with N. baluchestanic, the fruits, and seeds of N. ritchiana were stored for two years before they were subjected to the experiments.
The antimicrobial activity of N. baluchestanica has not been investigated previously; however, the antifungal effects of 80% methanolic extract of its closely related species, N. ritchiana, root and its crude extracts, including dichloromethane, butanol, petroleum ether, and ethyl acetate have been determined using agar tube dilution method. The authors reported MIC values of 62-80 µg.mL-1 and petroleum ether fraction was more effective in inhibiting the growth of fungi than others (15).
The methanolic extracts of the seed and fruit of N. ritchiana against various pathogens showed moderate to weak antimicrobial effects, with F. oxysporum being the most sensitive fungi to the extracts. However, no significant antimicrobial effect was seen between the fruit and seed extracts, and only the seed extract was effective against Enterococcus faeca and C. albicans (Author’s Unpublished Work). Similar to these results, N. baluchestanica showed modest antimicrobial activity, but its fruit extract was more efficient against the microorganisms than the seed extract.
The seed and fruit extracts of N. baluchestanica are more potent in scavenging DPPH free radicals and show higher efficacy against pathogenic microorganisms than N. ritchiana, thus making it a better candidate for possible future research and investments.
5.1. Conclusions
Hydroethanolic extracts of the fruit and seed of N. baluchestanica were analyzed for their antioxidant and antimicrobial potentials. The extracts demonstrated remarkable antioxidant effects, particularly the fruit extract. Moreover, the fruit extract could inhibit most of the tested pathogenic bacteria and fungi. It may be concluded that the same molecules were responsible for this plant’s antimicrobial and antioxidant properties. Based on this study, the fruits of N. baluchestanica may be prescribed as herbal medicine for the treatment and/or prevention of oxidative stress-related diseases. It is highly recommended that studies on this newly described palm species expand to document and appreciate this valuable endemic gene pool and open new horizons in various disciplines of biology, pharmacology, and the food industry.