1. Background
Hypericum androsaemum, commonly known as “tutsan,” is a small evergreen shrub found in damp woodlands and hedgerows in the Mediterranean Basin, South-Western Asia, North Africa, and other areas where it has been introduced (1). This plant, used in Europe as a traditional medicine, has large leaves with secretory structures that store naphthodianthrones. Many studies have been conducted on the leaves of this medicinal plant, which have revealed the presence of several flavonoids and phenolic compounds (2, 3). Additionally, this plant contains valuable secondary metabolites such as phenols, hypericin, and hyperforin; however, these metabolites can be affected by nitrogen nutrition or excessive amounts of metals (4).
Nitrogen (N) is a vital element for all living organisms and is a key macronutrient for plants. It is necessary to produce amino acids, proteins, and enzymes. A lack of nitrogen can lead to an increase in phenolic metabolites, which are antioxidants obtained from plants and also regulate the uptake of metals (5). Plants mainly use nitrate as their nitrogen source but can also absorb ammonium. Additionally, organic N forms are present in soil due to microbial activity or using fertilizers such as urea, which can affect how plants behave and change their metabolism (6).
Due to its significance in plant metabolism, a lack of N can lead to stunted growth and a lower yield of plants. This is often accompanied by a shift from N-based compounds to C-based ones, such as phenolic acids, flavonoids, and anthocyanins (4). While the effects of nitrate availability have been extensively researched, the profile of phenolic metabolites has only been examined for a few compounds or the total soluble phenols. Surprisingly, the impact of N deficiency on phenolics in H. androsaemum plants has yet to be explored.
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are molecules produced in plant tissues due to normal cell metabolism. These molecules, which include radicals such as superoxide anion (O2•-) and hydroxyl radical (OH•), as well as non-radical molecules like hydrogen peroxide (H2O2), can have both beneficial and harmful effects on plants (7). ROS are formed in response to stressors such as limited nitrogen availability, and plants have developed several mechanisms to protect themselves from the oxidative damage of these molecules (8). They include antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX), as well as non-enzymatic compounds like ascorbic acid, glutathione, and phenols (9).
Hypericin appears to be a significant secondary metabolite of the Hypericum species, as it was found in 27 out of 36 evaluated plants. This group of compounds includes five known substances: hypericin, pseudohypericin, protohypericin, protopseudohypericin, and cyclopseudohypericin (10). The role of these compounds in Hypericum plants has yet to be fully understood; however, they may act as deterrents to protect the plant from insects and other pests (11). For many years, Hypericum plants have been studied for their use in traditional medicine for various purposes (12, 13). The infusion of H. androsaemum leaves has previously been demonstrated to have scavenging effects against superoxide radicals (O2•), hydroxyl radicals (HO•), and hypochlorous acid (HOCl) (14). However, no information regarding other reactive oxygen species and reactive nitrogen species has been reported for this plant.
2. Objectives
This study aimed to investigate the response of the medicinal plant H. androsaemum to different nitrate doses as the only nitrogen source. For this purpose, we analyzed physiological parameters, main phenolic metabolites (total phenols, flavonoids, and phenylalanine ammonia-lyase (PAL)), secondary metabolites of hypericins, and gene expression related to its biosynthesis hyp-1 gene in plants cultured with nitrate. In addition, we investigated the plants’ oxidative stress and antioxidant response. The results of this research are discussed alongside other studies conducted on the Hypericum plant and other species treated with different nitrogen concentrations.
3. Methods
3.1. Plant Materials and Experimental Design
The H. androsaemum seeds were sterilized and placed in a growth chamber in the dark at 28 ± 1°C for 48 hours to initiate germination. The uniformly germinated seeds were then transferred to pots filled with sand and irrigated with full-strength Hoagland solution. The experiment was conducted in a growth chamber under controlled conditions, including a 12-hour day (6:00 am to 6:00 pm), a photon flux density of ~ 300 μmol.m-2.s-1 PAR at the leaf level, and a 25/20°C day/night temperature and relative humidity of ~ 60%. Plants that had been cultivated in the sand for four weeks were used for the experiment, which involved irrigating them with full-strength Hoagland solution (control, labeled as 1x in results), nitrate reduced to 1/3 (labeled as 1/3x in results), or no nitrate (labeled as no nitrate in results) for 14 days. After 14 days of exposure, individual plants were harvested for analysis. At the same time, fresh and dry masses (which had been dried at 75°C until a constant weight was reached) were weighed to calculate the water content of the tissue.
3.2. Antioxidant Enzyme Activities
Superoxide dismutase and APX activities were measured using a UV/VIS spectrophotometer. Fresh material (0.2 g FW/mL) was homogenized in 50 mM potassium phosphate buffer containing 1% insoluble PVPP (pH 7.0). The SOD activity was determined with a SOD assay kit (catalog number 19160, Sigma-Aldrich) according to the manufacturer’s instructions, and the APX activity was measured using the oxidation of ascorbic acid at 290 nm (15). The protein content in the reaction mixture was quantified by adding 20 μL of the supernatant to 980 μL of Bradford’s solution with BSA as standard and detection at 595 nm (16). The activities were reported as unit enzymatic activity per milligram protein (U/mg protein).
3.3. Phenylalanine Ammonia-Lyase Activity, Flavonoids, and Phenolics Content
The activity of PAL was assessed by measuring the production of t-cinnamic acid from phenylalanine using the HPLC method (7). The flavonoid content was determined based on the NaNO2-AlCl3-NaOH method (17), with absorbance measured at 510 nm and expressed as mg of catechin equivalent (CE). The total soluble phenols were measured in extracts prepared with 80% methanol using Folin-Ciocalteu phenol reagent (Sigma-Aldrich, Germany) detected at 750 nm and compared to gallic acid as a standard (15).
3.4. Specific Metabolites and hyp-1 Gene Expression
An analysis of hypericin, pseudohypericin, and hyperforin in Hypericum extracts dissolved in 60% ethanol was conducted using a modified version of non-aqueous capillary electrophoresis, as described by Dresler et al. (9). Quantification was done using authentic standards from Sigma-Aldrich.
The Easy Blue Absolute RNA Extraction Kit (iNtRON, South Korea) was utilized to extract total RNA from leaf tissues, following the instructions provided by the manufacturer. Electrophoresis in agarose gel (1.0%) was then used to check the integrity of the RNA, and a spectrophotometer (Nanodrop Eppendorf, Germany) was employed to determine its value. Additionally, A260/A280 ratio was calculated to estimate the purity of the RNA. The Power cDNA Synthesis Kit with gDNA Eraser PC5402 (iNtRON, South Korea) was used to reverse-transcribe the RNA into first-strand cDNA. Primers for the hyp-1 gene were designed Oligo 7. The qRT-PCR reactions (ABI, CA, USA) consisted of 0.4 μL of each primer along with 2 × Power SYBER Green PCR Master Mix (10 μL), diluted cDNA template (2 μL), and ddH2O (7.2 μL). The Applied Biosystem Step-one Plus System (ABI, CA, USA) was used to carry out the reactions according to the following amplification protocol: A first denaturation at 95°C for 5 minutes, followed by 40 cycles of 95°C and 60°C for 10 and 60 seconds respectively. Three repetitions (biological replicates) were considered for each gene in the experiment. Following Livak and Schmittgen (18), 2-ΔΔCT was employed as the relative quantification method.
3.5. Statistical Analysis
The experiment was repeated three times to ensure accuracy. Statistical analysis was conducted using GephPad Prism 9, and one-way ANOVA was used to compare the results. Duncan, multiple comparison tests, were applied, and a P-value of less than 0.05 was considered statistically significant.
4. Results
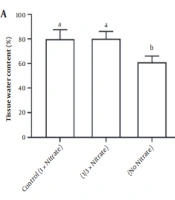
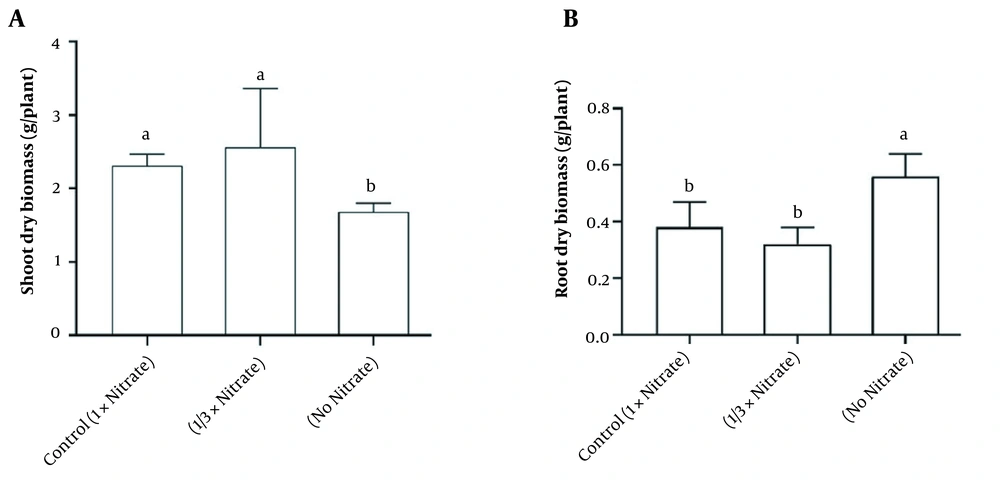
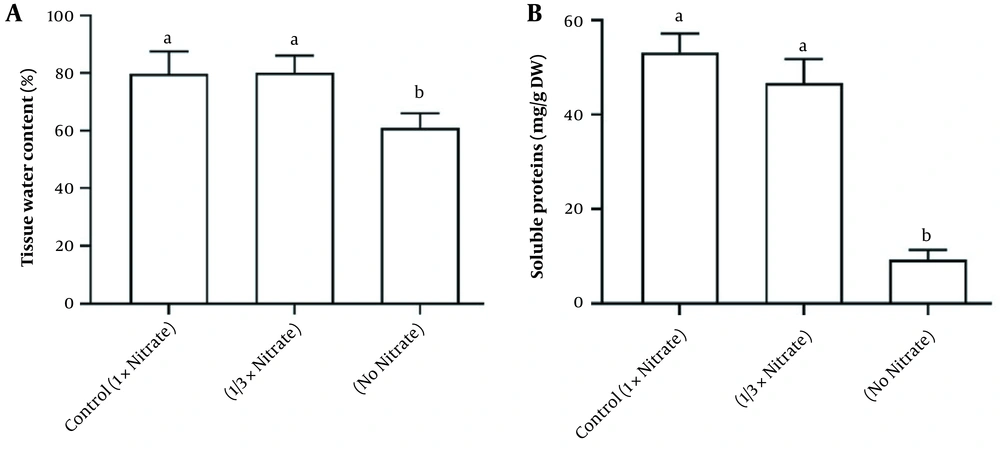
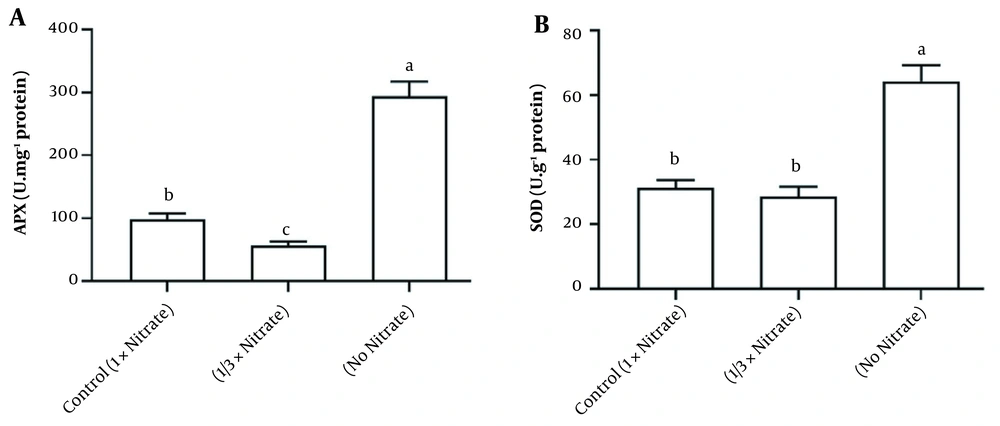
Nitrate deficiency caused a major decrease in the shoot biomass. Still, it did stimulate root biomass production (Figure 1). Water content and soluble protein content in the tissue were also reduced due to nitrate deficiency (Figure 2). Figure 3A and B demonstrates the activities of antioxidant enzymes in H. androsaemum exposed to different nitrate levels. Significant increases were found in the activities of SOD and APX in no nitrate condition. Moreover, in 1/3x nitrate condition, APX activity was significantly lower than the control group, but SOD activity was similar to the control group.
A, Ascorbate peroxidase; and B, Superoxide dismutase (SOD) activity of Hypericum androsaemum plants after 14 days of treatment with various nitrogen doses. The data are presented using means ± SD. Columns by the same letter were not significantly different according to Tukey’s test (P < 0.05).
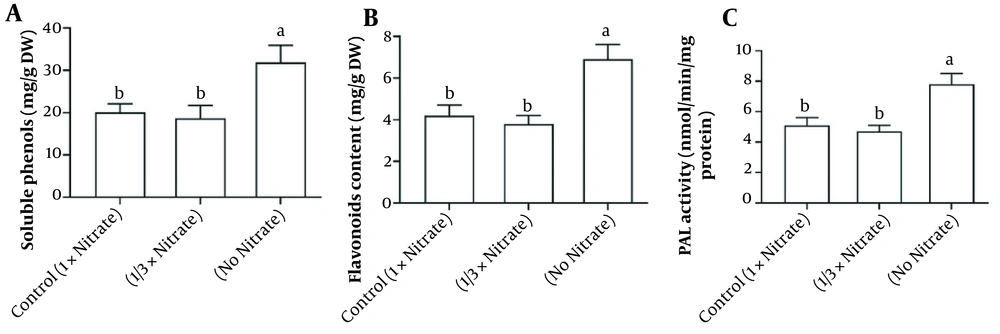
After 14 days of treatment, the soluble phenols and flavonoids had increased accumulations under nitrate deficiency compared to the control group by 60% and 64%, respectively. However, reduced nitrate availability (1/3x) had no significant effects on soluble phenols and flavonoid content compared to 1x nitrate level. PAL enzyme activity increased by nitrate deficiency, but in 1/3x, nitrate was equal to the control group (Figure 4).
A, Soluble phenols; B, Flavonoids; and C, Phenylalanine ammonia-lyase (PAL) enzyme activity of Hypericum androsaemum plants after 14 days of treatment with various nitrogen doses. The data are presented using means ± SD. According to Tukey’s test (P < 0.05), the column by the same letter was not significantly different.
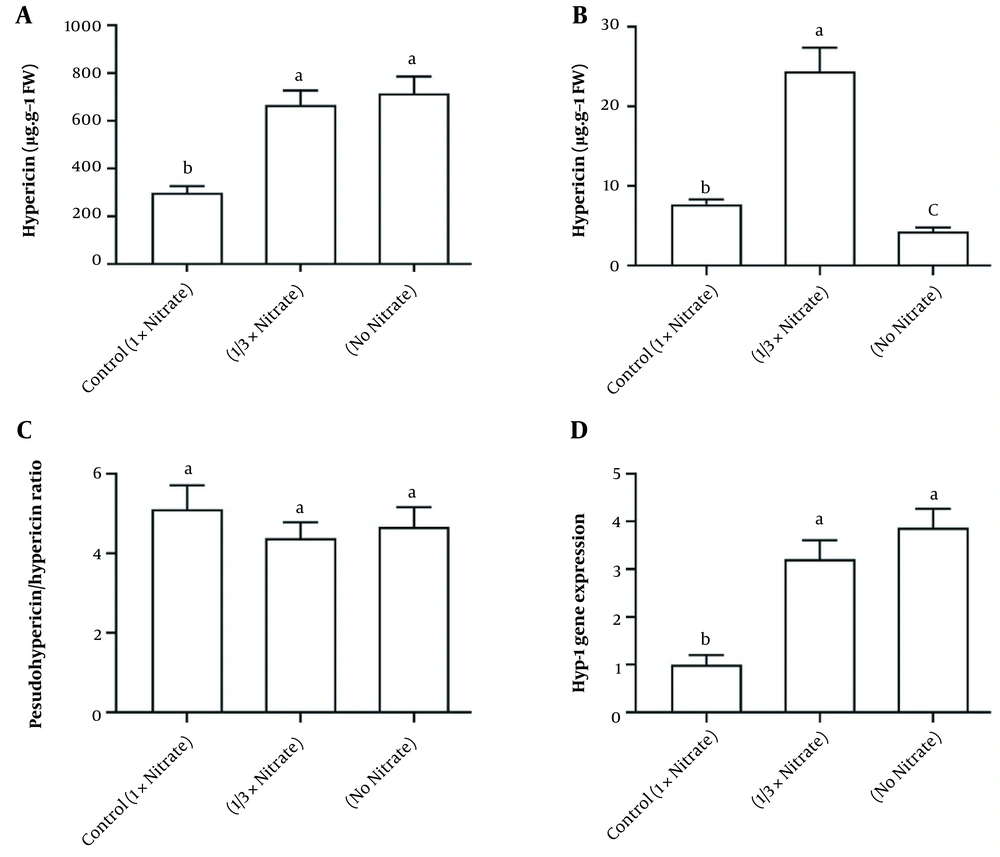
When nitrate availability was decreased (1/3x), there was a significant increase in hypericin, pseudohypericin, and hyperforin content (Figure 5). However, nitrate deficiency led to an increase in hypericin and pseudohypericin levels. Still, it resulted in a decrease in hyperforin, while the pseudohypericin/hypericin ratio was not affected by either nitrate deficiency or reduced nitrate availability. The expression of the hyp-1 gene in the leaves of H. androsaemum treated with different levels of nitrate is shown in Figure 5D. In both the 1/3x and no nitrate groups, the expression of the hyp-1 gene was significantly increased.
A, Hypericin; B, Hyperforin; C, Pseudohypericin/hypericin ratio; and D, Expression of hyp-1 gene of Hypericum androsaemum plants after 14 days of treatment with various nitrogen doses. The data are presented using means ± SD. According to Tukey’s test (P < 0.05), the column by the same letter was not significantly different.
5. Discussion
The applied nitrogen had different effects on the morphology of the plants, and N deficiency caused a decrease in shoot biomass and an increase in its roots. This is consistent with a previous study demonstrating that long-term NO3 starvation decreased shoot biomass but increased root growth (7). Moreover, during N deficiency in Hypericum perforatum, a decrease in shoot biomass but an increase in root growth was observed (7). It has been suggested that in N deficiency, the enlarged root system intensifies of nitrogen absorption from the environment (19), where nitrogen mobilization from internal sources (such as phenylalanine deamination by PAL enzyme) can play an important role (7).
According to the results, tissue water content and soluble proteins decreased in nitrogen deficiency. This is in agreement with previous research on chamomile plants demonstrating that proteins were suppressed more by nitrate deficiency (20). However, organic nitrogen metabolites such as proteins are always depleted or tend to be depleted in N-deficient plants (21). A sharp decrease in protein content in nitrate deficiency indicates its effect on protein biosynthesis. Nitrate is essential for plant species’ optimal growth and basic physiology.
Nitrogen deficiency, which affects the oxidative balance, can be considered an abiotic stress factor. For example, increased H2O2 production has been reported in nitrogen deficiency in Arabidopsis roots (22) and chamomile shoots and roots (7). In Hypericum spp., significant variation in the activities of APX, CAT, and SOD has been observed, and these enzymes have been demonstrated to play an essential role in ROS scavengers for normal plant growth (23). Therefore, according to our results, these effects on growth parameters were predictable. In this study, N deficiency significantly affected the corresponding antioxidant enzymes (APX and SOD). In agreement with our research, increased activity of SOD and APX was observed in rice and cucumber plants under N deficiency (24). Cui et al. also observed H2O2 accumulation in Hypericum (25).
In the last decades, phenolic metabolites with many positive health effects have received much attention, and they are demonstrated to be a broad and structurally variable group of secondary metabolites (26). PAL enzyme is a key step in the biosynthesis pathway of phenols, which is regulated during N deficiency (27). Under N deficiency, an increase in its activity was observed in shoots, which was corroborated by growth changes. The observed increase in the PAL activity in N-deficient plants is consistent with previous studies (19, 21). However, a high level of soluble phenols was observed in no-nitrate treatments, which is another indication of stimulation of phenolic metabolites in response to N deficiency. In agreement with our results, total soluble phenols in spinach shoots (28) and H. perforatum plant cultures increased with nitrogen depletion (29). Due to their high antioxidant activity, flavonoids are phenols’ most intensively studied compounds (30). Unfortunately, their accumulation in shoots is less studied because they are mostly present in flowers. Flavonoids had the highest accumulation under nitrate deficiency. In other species, these metabolites also respond to nitrogen deficiency by increasing their accumulation (31). Due to the abundance of phenolic metabolites, they can also provide antioxidant protection due to their high antioxidant properties (32).
Pseudohypericin, hypericin, and hyperforin are among hypericins, which are important therapeutic secondary metabolites produced by H. androsaemum plants. Our research demonstrated that nitrogen supply to H. androsaemum plants could have a profound effect on the content of these metabolites in shoots. The present research is in agreement with previous studies in which hypericin content was higher than hyperforin in laboratory-cultured shoots (33), while sand-cultured H. perforatum shoots contained more hyperforin than hypericin (9). Also, in our study, the amount of pseudohypericin was more abundant than hypericin, which was consistent with the results of Briskin et al. (6). Our results demonstrated that the decrease in nitrate availability caused a significant increase in the content of hypericin types. On the contrary, nitrate deficiency stimulated hypericin and pseudohypericin accumulation, but hyperforin content decreased. These findings are consistent with previous studies where reduced nitrogen availability increased total hypericin content (6, 29). Our study demonstrated that nitrate deficiency had the greatest effect on pseudohypericin, hypericin (increase), and hyperforin (decrease). Hitherto, there has yet to be a report on the effect of nitrogen on hyperforin content that can be discussed in detail.
Analysis of the putative gene in hypericin biosynthesis hyp-1 has demonstrated a relative association with pseudohypericin and hypericin rather than with hyperforin content, mainly observed in treating nitrate deficiency. It was previously reported that the function of this gene is not limited to hypericin biosynthesis (29), which can be seen in the present study. Although the details of the biosynthetic pathway for the production of hypericin and pseudohypericin are not fully understood, it has been demonstrated that this reaction is primarily controlled by mass action, and the levels of early intermediates in the metabolic pathway may be modulated in response to nitrogen supply. It has also been suggested that hypericin and pseudohypericin are products of anthracoid metabolism originating from the polyketide pathway (34). Currently, studies are being conducted on the biosynthesis pathway of these metabolites with an emphasis on a better understanding of how effective genes and environmental factors such as nitrogen supply regulate this pathway.
It can be concluded that in H. androsaemum plant, N deficiency acts as abiotic stress, which increases antioxidant enzymes, phenolic compounds, and metabolites of hypericin and pseudohypericin and the expression of hyp-1 gene related to these metabolites’ biosynthetic pathways. Therefore, the present study supports the role of hypericin and pseudohypericin metabolites in plant defense systems. Based on these data, in this medicinal plant, higher amounts of secondary metabolites are formed in N deficiency, which are beneficial for health in addition to the plant’s defense mechanisms. Currently, more studies are needed on regulating the biosynthesis of hypericin and pseudohypericin by environmental factors such as nitrogen supply.