1. Background
Acute kidney injury (AKI) is a serious subject in clinics, so the sick are at high risk of cardiovascular incidents, chronic or ending phase renal problems, and death (1). Renal ischemia-reperfusion (I/R) harm is one of the main causes of AKI. During patient improvement, a transient reduced kidney blood flow attended by reperfusion occurs in several clinical situations containing sepsis, cardiac surgery, and renal transplantation (2-4). Although the molecular mechanisms that drive renal I/R injury are yet not entirely comprehended, it is well found that oxidative stress plays a vital role in renal I/R pathophysiology (5).
Diminished oxygen and nutrient supply, accumulation of toxic products in the cells, and the release of pro-inflammatory cytokines have important performance in renal I/R (6). In addition, the overproduction of reactive oxygen species (ROS) may be responsible for tubular epithelial cell damage by apoptosis, necrosis, and ferroptosis cell death types (7-9). Hence, several studies have demonstrated improved symptoms when oxidative stress is inhibited (8, 10, 11). So far, numerous candidate therapeutic agents have been reported to have protective/ameliorative effects on kidney I/R harm. Studies showed that ferritin (6), propofol (8), pachymic acid (from Poria cocos) (12), mangiferin (13), and atorvastatin (14) could reduce, ameliorate, or prevent renal I/R in animal models, by inhibitory effects on inflammation and oxidative stress. Because of the great importance of this issue, research is still ongoing to find effective compounds to protect kidneys from I/R.
Acacetin (5,7-dihydroxy-4-methoxy flavone) is a natural flavonoid in various plants. It is known to possess multiple useful efficacies, containing anti-inflammatory, anti-cancer, anti-obesity, anti-diabetic, protective effects (lung injury, hepatic protection, cardiac protection, and neuroprotection), and anti-microbial activities (15, 16). Some data clearly illustrate the helpful involvement of ACA in oxidative stress and inflammation-related situations. ACA administration also improved complications caused by I/R, including inhibition of apoptosis and autophagy in cardiomyocytes under I/R (17) and suppression of inflammation in cerebral I/R (18). Moreover, our previous study showed that ACA had liver protection against I/R-induced hepatitis through its anti-oxidative and anti-inflammatory effects (19).
2. Objectives
In this investigation, we aimed to further check the molecular mechanisms of the ameliorative effect of ACA in kidney I/R harm.
3. Methods
3.1. Animal Model of Renal I/R Injury
The animal care and protocols were approved by the Ethics Committee of Kermanshah University of Medical Sciences (ethics certificate No. 1396.348). The modeling of I/R in the kidney was described previously (19). Male Balb/c mice were kept on a 12/12 h light/dark cycle at 23 ± 2°C and had free access to food and water. Animals were randomly divided into four groups (n = 7): Sham-operated group (laparotomy: Yes, renal artery ligation: No; 0.1% DMSO); I/R group (laparotomy: Yes, renal artery ligation: No); I/R treated with 50 mg/kg ACA group; control group (laparotomy: No, renal artery ligation: No; 0.1% DMSO). Ischemia was created by ligation of the left renal artery for 60 minutes; it was followed by reperfusion. A day after surgery, the mice received DMSO or ACA intraperitoneally daily for four consecutive days. Finally, blood and tissue sampling was conducted 24 h after the last injection, so all animals were sacrificed on day 5. The isolated serum samples and kidney tissues were kept at -80°C until experiments. All the I/R, laparotomy, and sacrifice operations were done when the mice were maintained in deep anesthesia, accomplished by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg).
3.2. Measurement of Serum Creatinine
Blood sera were collected by centrifuging the samples and stored at -80°C until analysis of creatinine level. The creatinine concentrations were analyzed with a commercial kit (Bioassay System, USA) according to the manufacturer’s instructions.
3.3. Superoxide Dismutase and Glutathione Peroxidase Activities
Activities of superoxide dismutase (SOD) and glutathione peroxidase (GPx) enzymes were detected by commercial kits (SOD activity and GPx activity, Kiazist, Iran) according to the manufacturer’s directions.
3.4. Real-time Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted after homogenizing the whole kidneys using TRIzol reagent and a standard RNA extraction protocol (Takara Bio, Inc.). The cDNA was synthesized using the PrimeScript First Strand cDNA Synthesis Kit (TaKaRa Bio, Inc.). The reactions were performed using SYBR Green Master Mix (TaKaRa Bio Inc.) and Corbett Rotor-Gene 6000 thermocycler. Beta-actin housekeeping gene was considered an internal control to normalize the expression levels of the samples, and relative expression levels of the target genes were calculated using the 2-ΔΔCt formula. The sequence of primers is given in Table 1.
| Gene | Sequence (5’ to 3’) | |
|---|---|---|
| F | R | |
| Thioredoxin 1 (Trx1) | CCCTTCTTCCATTCCCTCT | TCCACATCCACTTCAAGGAAC |
| Heme oxygenase-1 (Ho-1) | CCTTCCCGAACATCGACAGCC | GCAGCTCCTCAAACAGCTCAA |
| Nuclear factor (erythroid-derived 2)-like 2 (Nrf-2) | CAGCATGATGGACTTGGA | TGAGACACTGGTCACACT |
| Interleukin-1 beta (IL-1β) | TGTAATGAAAGACGGCACACC | TCTTCTTTGGGTATTGCTTGG |
| Interleukin-6 (IL-6) | CTGCAAGAGACTTCCATCCA | AGTGGTATAGACAGGTCTGTTG |
| Interleukin-18 (IL-18) | ACTGTACAACCGCAGTAATACGC | AGTGAACATTACAGATTTATCCC |
| Interleukin-10 (IL-10) | GGTTGCCAAGCCTTATCGGA | ACCTGCTCCACTGCCTTGCT |
| Tumor necrosis factor-alpha (TNF-α) | GGGGCCACCACGCTCTTCTGTC | TGGGCTACGGGCTTGTCACTCG |
| cyclooxygenase-2 (COX-2) | ACACACTCTATCACTGGCACC | TTCAGGGAGAAGCGTTTGC |
| Actin beta (Actb) | CACTTTCTACAATGAGCTGCG | CTGGATGGCTACGTACATGG |
Primers Used for Real-time Reverse Transcription Polymerase Chain Reaction
3.5. Enzyme-linked Immunosorbent Assay
Kidney tissues from each group were collected and homogenized using RIPA solution (RIPA Lysis and Extraction Buffer, Thermo Scientific™, USA). The homogenate was centrifuged at 10,000 × g for 30 min at 4°C, and the supernatant was collected. The levels of interleukin-10 (IL-10) (Elabscience Biotechnology Inc., USA), cyclooxygenase-2 (COX-2) (MyBioSource, USA), and tumor necrosis factor-alpha (TNF-α) (MyBioSource, USA) were measured using mouse enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s protocols.
3.6. Statistical Analysis
One-way ANOVA tested data for each group and post-hoc LSD to detect differences between groups. Data are expressed as mean ± standard error of the mean (SEM), and P-value < 0.05 was considered statistically significant.
4. Results
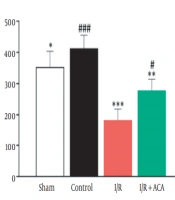
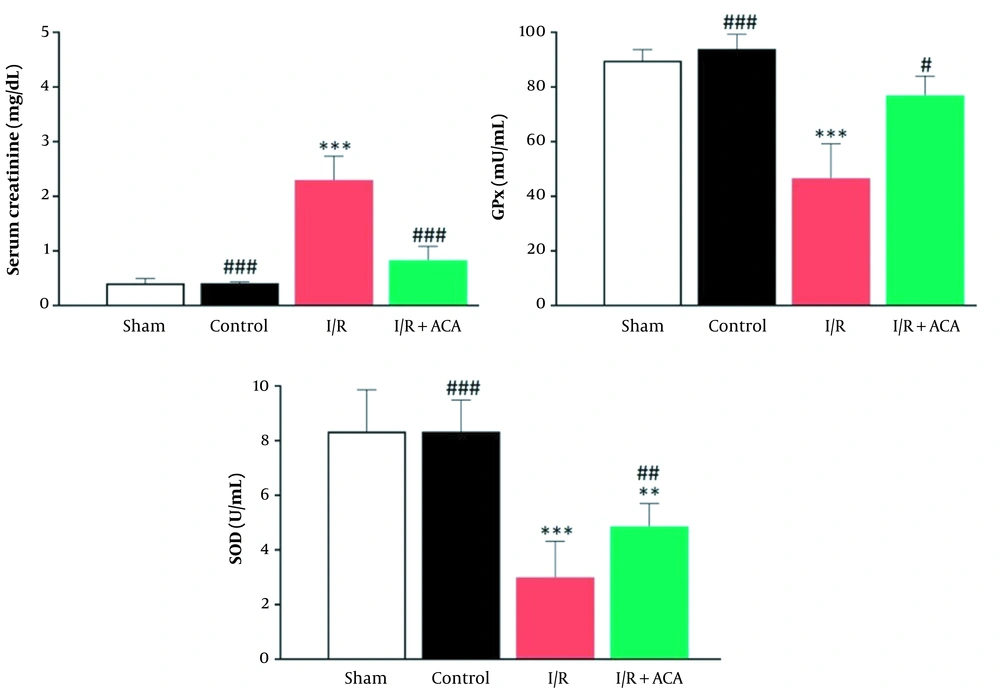
Blood creatinine level in the I/R group following 24 h reperfusion was increased in comparison with the control group (P < 0.001), while its level was significantly lower in the ACA-treated I/R mice than the I/R group (P < 0.001). Moreover, there was no obvious difference in creatinine value between the control and vehicle solution-treated groups (Figure 1A). Outcomes of oxidative stress markers revealed that SOD activity in the I/R-treated ACA group had remarkably higher levels (P < 0.01) than in the I/R animals (Figure 1B). There was no significant difference in the ACA-treated I/R group rather than the control. GPx activity of I/R mice was lower than the control group; however, it showed an increased value in ACA-treated ones (P < 0.05) (Figure 1B). Control and sham-operation groups had similar GPx activity.
Acacetin (ACA) inhibited renal ischemia/reperfusion (I/R) injury. Blood and kidney samples were collected after 24 h reperfusion. A, Serum levels of creatinine; B, Enzyme activity of superoxide dismutase (SOD) (U/mL) and glutathione peroxidase (GPx) (U/mL) in plasma of mice. Data were expressed as mean ± standard error of the mean (SEM). * P < 0.05, ** P < 0.01 compared to control group; ## P < 0.05, ## P < 0.01 compared to I/R group
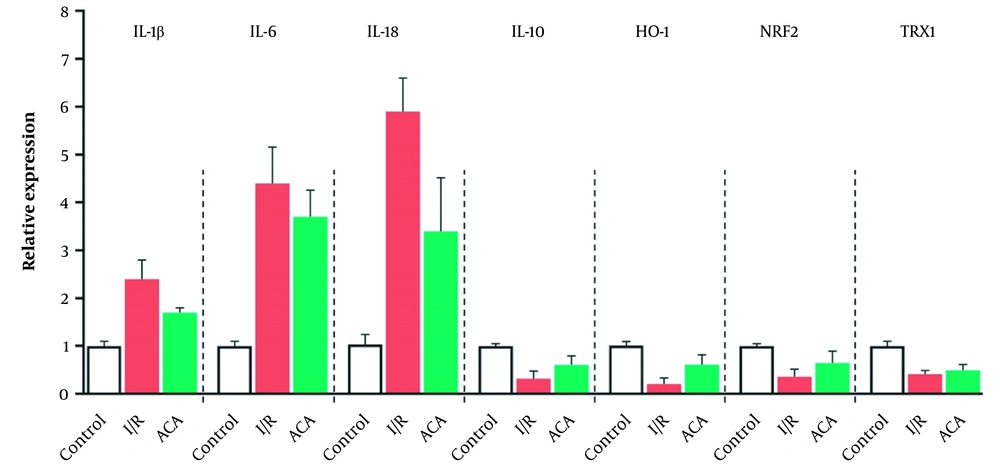
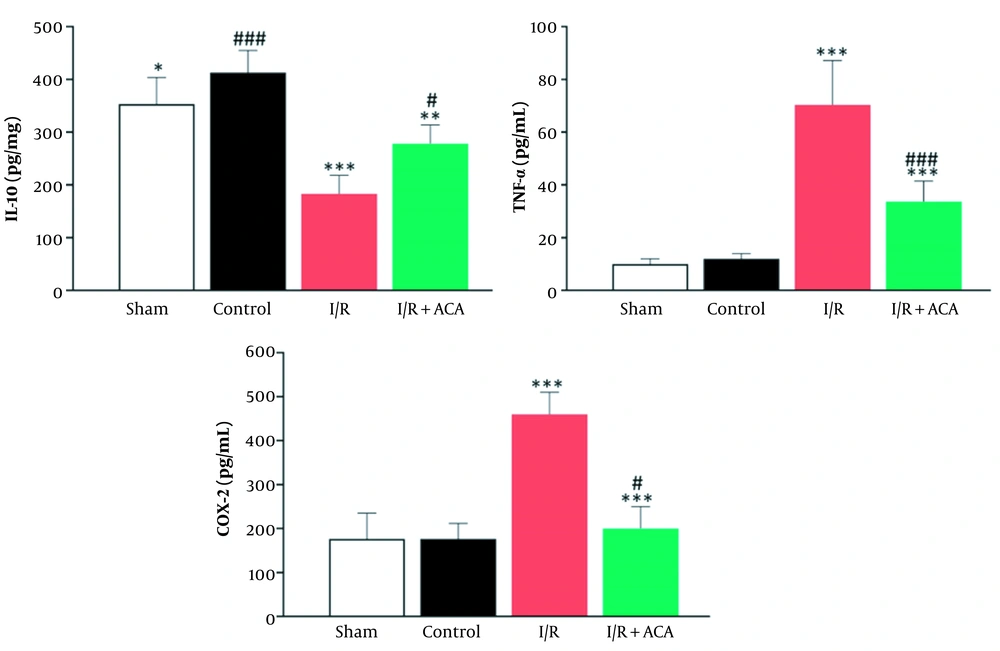
As shown in Figure 2, renal I/R injury led to a notable decline in IL-10 (P < 0.05) levels than in the sham group, while ACA significantly reversed this change caused by I/R. Increased mRNA expression of interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and interleukin-18 (IL-18) in the I/R group in comparison with the sham group, confirming that inflammation was involved in the I/R-induced injuries. This increase was reversed by ACA treatment (Figure 3). We also detected the expression of genes involved in the response to oxidative stress. After I/R, the mRNA expression of heme oxygenase-1 (Ho-1), nuclear factor (erythroid-derived 2)-like 2 (Nrf-2), and thioredoxin 1 (Trx1) was significantly decreased compared to the sham group, and the expression level of these genes was increased by ACA treatment (Figure 3). As shown in Figure 2, after reperfusion for 24 h, the expression of TNF-α was increased, and the expression level was increased approximately 7-fold in I/R relative to the control group. After I/R, the level of COX-2 was increased while the level of this protein was significantly reduced following ACA treatment (Figure 3).
Acacetin (ACA) decreased oxidative stress caused by ischemia/reperfusion (I/R) injury. A, Interleukin-10 (IL-10) increased following I/R injury. Down-regulation of IL-10 protein level in kidney tissue of mice evaluated by enzyme-linked immunosorbent assay (ELISA) after renal I/R; B, Oxidative stress evaluated by real-time reverse transcription polymerase chain reaction (real-time RT-PCR). The expression levels were normalized to the actin beta (Actb). Data are presented as mean ± standard error of the mean (SEM). * P < 0.05; ** P < 0.01 compared sham group, # P < 0.05; ## P < 0.01 compared I/R group
5. Discussion
This study aimed to determine whether ACA could protect kidneys from I/R injury. Outcomes showed that ACA remedy could alleviate I/R-induced changes in renal function and reduce apoptosis and oxidative stress in mice. Previous studies have shown that I/R has damaging consequences such as increased oxidative stress, elevated inflammatory mediators and chemokines levels, and raised cell death signaling (20, 21). So, it can be said that reducing cell death and oxidative stress caused by I/R can protect organs from subsequent injuries; ACA, via affecting these processes, can be assumed as a treatment case for I/R. We showed that ACA enhanced the antioxidant capacity in I/R mice by elevating SOD and GPx, two important antioxidant enzymes. This agrees with the outcomes of Liu et al.’s study (17). They showed a significant decrease in SOD activity in hypoxia/reoxygenation, but it was increased with ACA pretreatment.
The real-time reverse transcription polymerase chain reaction (real-time RT-PCR) results displayed that ACA protected cells from apoptosis. In a study by Li et al., propofol attenuated I/R in kidneys (8). Results showed that pretreatment with the drug restored renal function and less tubular apoptosis than untreated I/R rats. We also observed enhanced expression of pro-inflammatory factors IL-1β, IL-6, and IL-18 in renal I/R, which was mitigated after ACA treatment. An investigation displayed that the mRNA expression of caspase-1, IL-1β, and IL-18 was remarkably elevated in hypoxic HK-2 cells (a proximal tubular cell line) than in those in normoxic HK-2 cells (22). Results of a study indicated that ACA protected H9c2 cardiomyocytes from hypoxia/reoxygenation injury by enhancing autophagy through activating the PI3K/Akt/mTOR signaling pathway and improving the autophagy proteins (Beclin 1, LC3-II, and p62) expression (17).
Our results also demonstrated that I/R injury resulted in downregulated expression and production of IL-10, and a reverse trend was seen in the ACA-I/R treated group in comparison with the sham group. IL-10 effectively ameliorates I/R injuries through its anti-inflammatory and anti-apoptotic properties. It has been reported that in a murine model of chronic kidney damage, IL-10 inhibited apoptosis caused by inflammation (23). The results of a study showed that compared to wild-type mice with renal I/R, in IL-10 knockout mice with I/R, blood urea nitrogen, creatinine, mRNA expression of kidney damage molecule-1 (Kim-1), IL-1β, IL-6, and IL-18, Bax expression, and cleaved caspase 3 showed an increasing trend (24).
Our results also showed that I/R resulted in a decrement in expression levels of Ho-1, Nrf-2, and Trx, while ACA reversed these reductions. In line with our results, Wu et al. reported that ACA treatment elevated Nrf-2, Ho-1, and IL-10 and reduced superoxide dismutase 1 and 2 declines induced by I/R (25). Also, ACA repressed the Toll-like receptor (TLR)-4 and IL-6 release induced by hypoxia/reoxygenation hurt (25). A study showed that intraperitoneally injection of pachymic acid for three days had a protective effect on renal I/R injury in mice, which may depend on ferroptosis dissuasion in the kidneys. These results demonstrated that pachymic acid remedy declined serum creatinine, blood urea nitrogen, and the expression levels of malondialdehyde and COX-2. Further, it enhanced glutathione and the protein and mRNA levels of ferroptosis-related proteins, glutathione peroxidase 4 (GPx4), solute carrier family seven member 11 (SLC7A11), NRF-2, and Ho-1 in the kidney (12).
Our previous studies found that ACA administration could decrease the renal I/R-increased serum levels of creatinine, urea, and MDA (26). ACA also decreased nitric oxide (NO) secretion, whereas total antioxidant capacity augmented in comparison with the I/R group. In hepatitis caused by renal I/R, ACA resulted in a decreasing trend in liver enzymes alanine transaminase and aspartate transaminase, as also TNF-α, IL-1β, and TLR4 proteins (19). ACA protected the lipopolysaccharide-induced acute lung damage in mice by decreasing TNF-α and IL-1β levels in tissue and elevating Nrf-2 and Ho-1 at mRNA and protein expression levels (27). Also, ACA showed an anti-inflammatory effect by reducing the expression activity of mRNA and protein levels of COX-2 and nitric oxide synthase. It also prevented the degradation and phosphorylation of IkBa, therefore inhibiting the translocation of nuclear factor kappa B (NF-κB) (28). ACA reduced pulmonary inflammation, edema, myeloperoxidase, SOD, and Ho-1 activity. Intraperitoneal injection of 25 mg/kg to cerebral I/R injury model in C57BL/6 mice caused a reduction in their neurological function scores and cerebral infarction volumes. ACA remedy decreased the pro-IL-1β, IL-1β, IL-6, NF-κB, TLR4, Iba1, NLRP3, and caspase-1 expression (18).
Our results exhibited that ACA was able to improve the events caused by kidney I/R. From existing data, it can be said that ACA can prevent or ameliorate the consequences of I/R; however, the mechanisms of its effectiveness still need further study.