1. Background
Nanoparticles are atomic assemblies with crystalline or amorphous structures that exhibit unique physical and chemical properties (such as melting point, thermal and electrical capabilities, catalytic activity, and optical, magnetic, and electrical properties). These properties make nanoparticles of great interest in various fields of research (1). Nanoparticles have a high surface-to-volume ratio due to their small size, which makes the surface atoms more active than the inner atoms, affecting the sample’s properties (2). This property of nanoparticles has resulted in the formation of technological sciences, as materials on the nanoscale begin to change their behavior, and the behavior of atoms on the surface overcomes the bulk behavior, resulting in the quantum physics phenomena (3). Nanoparticles have been found to be promising candidates for a wide range of applications (including catalysis, imaging, medical, and environmental applications) because of their unique structure, size, shape, and reactivity (1). Several physical, chemical, and biological ways are available for producing nanoparticles. Despite their popularity, physical and chemical methods are associated with a few issues, such as being expensive, needing a lot of energy, causing contamination, having low efficacy, and having restricted use in biomedicine (4). Biological methods have received more attention than other methods for the production of nanoparticles due to their faster speed, lower energy and lower cost requirements, the ability for large-scale production, and minimal environmental impact (5). Several studies have proven that bacteria, yeast, fungi, and plants can be used to create a variety of metal nanoparticles through biological methods. When exposed to metal ions, these organisms absorb them into or onto their cell walls, forming nanoparticles (6). Generally, biologically created nanoparticles have features such as a large surface area, small size, and high monodispersity, as well as improved stability and control of their formation (7). The biosynthesis of nanoparticles is more efficient with fungi than other biological methods because of their greater ability to collect metal ions. Fungi are able to make environmentally friendly enzymes (6). Copper carbonate nanoparticles have many practical uses, from fungicides and insecticides to catalyzing organic processes and desulfurizing crude oil (8). In addition, copper carbonate nanoparticles are efficient and reliable substitutes for gold and silver nanoparticles due to their electrical and thermal conductivity (8). Despite comprehensive research into the biogenesis of metal nanoparticles, little is understood regarding the biogenesis of metal carbonates and their capacity. Ureolytic organisms are capable of precipitating carbonates, a phenomenon that is well-documented. Microorganisms such as filamentous fungi use amidohydrolases to hydrolyze urea, resulting in the formation of ammonium and carbonate, which causes carbonate precipitation. The released carbonate then reacts with the metal ion and results in metal carbonates (9).
2. Objectives
Considering the potential of fungal isolates in nanoparticle biosynthesis, this study aimed to investigate the possibility of generating copper carbonate nanoparticles under a cell-free extract (CFE) strategy, as well as to assess the antibacterial activity of the nanoparticles created.
3. Methods
3.1. Enrichment of Fungal Isolates
Ten surface water samples were collected from different locations around Saral Divandareh City in Kurdistan Province. Samples were transferred to 100-mL tubes and centrifuged at 5000g for 10 minutes. To isolate copper-resistant fungi, 500 µL of the aqueous solution was inoculated into a PDA medium supplemented with copper chloride stock at a concentration of 25 mM. Streptomycin was used as a selective agent in culture media at a concentration of 35 mg/L to inhibit bacterial growth (10). The culture plates were incubated for a period of 5 to 14 days at a temperature of 25°C. The fungal isolates were purified with the single spore and hyphal tip technique on the PDA medium (11).
3.2. Quantitative Urease Activity of Selected Fungi
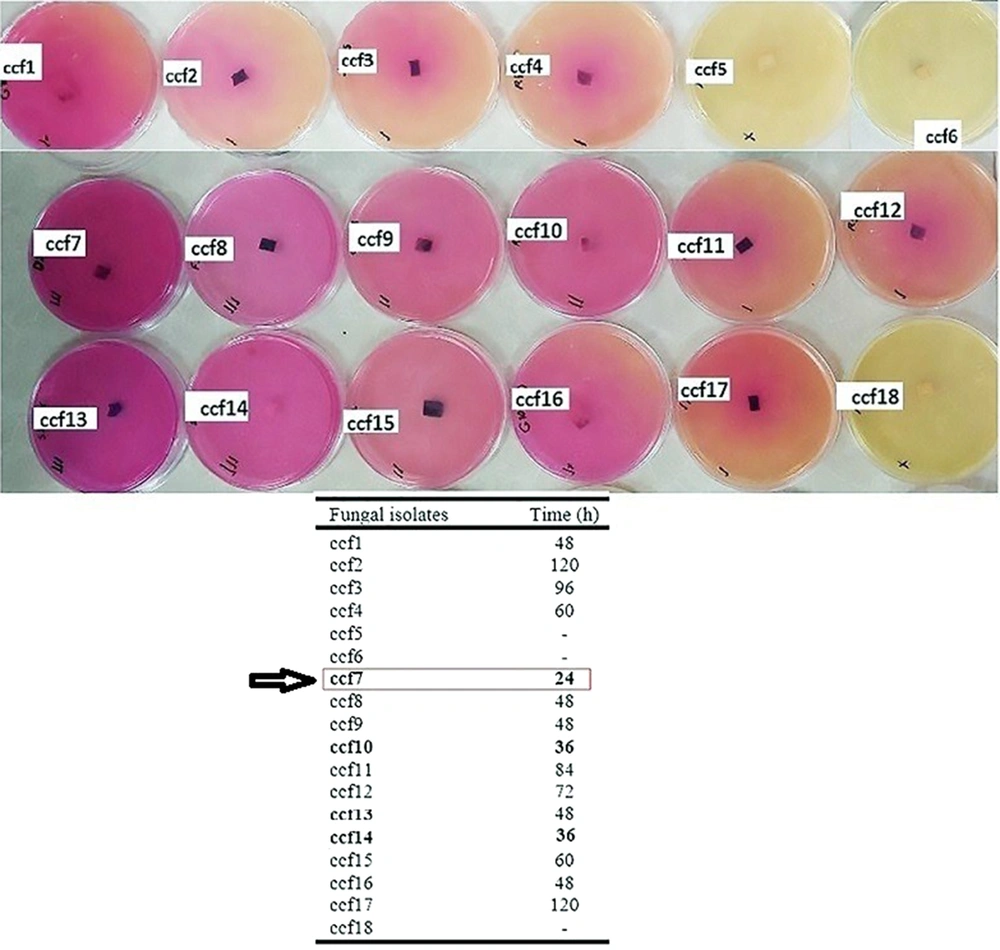
The procedure described by Martuscelli et al. (12) was applied to determine the qualitative activity of the urease enzyme. To evaluate the urease activity of the fungal isolates, a mycelial disc with a diameter of 5 mm was taken from the margin of a 5 to 14-day-old fungal culture and inoculated on a urea agar-based culture medium (CHRISTENSEN Agar) containing 2% sterilized urea, using 0.22 µm pore filter membranes. The inoculated cultures were incubated for 120 hours at 25°C. The culture plates were tested every 12 hours to evaluate the activity of the urease enzyme based on the color change of the culture medium. A urea agar-based culture medium containing 2% urea without any fungal isolates was used as a control. Fungal isolates that are highly active in urease production can make the medium culture turn a deep pink within 24 hours. Fungi with moderate urease activity may require up to 3 days to change the color of the medium to pink. Fungal isolates with low urease activity change the color of the medium to light pink, which normally takes 72 to 120 hours. No color changes were observed in fungi that lacked urea hydrolyzing capacity after 120 hours.
3.3. Biosynthesis of Copper Carbonate Nanoparticles by Ureolytic Fungi
The mycelium-free extract from the chosen fungal isolate was used as a biocatalyst in the production of copper carbonate nanoparticles. For this purpose, a fungal disc with an approximate diameter of 10 mm, taken from a 7-day culture of the selected fungal isolate, was transferred to 250-mL Erlenmeyer flasks containing 50 mL of urea-modified AP1 medium (13). The medium contained 40 mM urea, 4 mM K2HPO4.3H2O, 0.8 mM MgSO4.7H2O, 0.2 mM CaCl.6H2O, 1.4 mM NaCl, 0.009 mM FeCl3.6H2O, 0.014 mM ZnSO4.7H2O, 0.018 mM MnSO4.4H2O, and 0.016 mM CuSO4.5H2O with a pH of 5.8 and was incubated for 7 days in a rotary shaker at 25°C and 150 rpm. The obtained fungal extracts were then cultured in sterile deionized water for 5 days after being separated by centrifugation (4000g for 30 minutes) and filtration through 0.45-µm membrane filters. Finally, CFEs from selected fungal isolates were used to generate biological carbonate for the fabrication of copper nano-carbonate. For this purpose, 50 mL of mycelium-free extracts were added to 250 mL Erlenmeyer flasks. The reaction mixture was then treated with copper chloride stock at final concentrations of 25 mM, 35 mM, 40 mM, and 45 mM. The inoculated flasks were kept in a dark environment in a rotary shaker at 25°C. Using spectroscopic and electron microscopic techniques, the properties of copper carbonate nanoparticles synthesized in the bioconversion reaction mixture were examined.
3.4. Characterization of Copper Carbonate Nanoparticles
Copper carbonate nanoparticles were purified by passing the CFE of the chosen fungal isolate through 0.22-μm sterile syringe filters. The sample was then centrifuged at 12 000g for 15 minutes at 4°C. The copper carbonate nanoparticle precipitate was washed 3 times with sterile deionized water (14). Then, the collected copper carbonate nanoparticles were freeze-dried for 24 hours (Christ Alpha 1-2Dplus, Germany). The size and shape of the copper carbonate nanoparticles were determined using techniques such as field emission scanning electron microscope (FESEM; TESCAN Mira 3-LMu, Czech Republic) and dynamic light scattering (DLS; SZ-100, HORIBA, Japan). A histogram of the particle size distribution was created using ImageJ; then, the average particle size was calculated. Fourier transform infrared spectroscopy (FTIR) on the Bruker Vector 22 FT–IR Spectrometer was performed to investigate the functional groups involved in the synthesis of copper carbonate nanoparticles. Copper carbonate nanoparticles can be analyzed for their chemical composition using energy-dispersive X-ray spectroscopy (EDX; Mira 3-LMu).
3.5. Identification of the Fungal Isolate
The chosen fungal isolate was identified through macroscopic and microscopic examinations, as well as molecular analysis. The PDA plate containing the selected fungus was visually inspected after 5 days at 25°C. By creating a slide from the culture and examining it under an OLYMPUS BX51 optical microscope, the microscopic characteristics of the chosen isolate were able to be observed and analyzed. The genomic DNA of the fungus was extracted using the phenol/chloroform technique and glass beads (15). To amplify the internal transcribed spacer (ITS) regions (18S SSU rRNA-ITS1-5.8S-ITS2-28S LSU rRNA), polymerase chain reaction (PCR) was performed using primers ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC) to create a fragment of approximately 550 - 600 base pairs (bp) (16). The PCR product was purified and then sequenced with an automated sequencer (Macrogen Inc, Seoul, Korea). The obtained sequences were compared with other similar sequences using the BLASTN algorithm. The resulting sequences were deposited in GenBank, and an accession number was assigned.
3.6. Antibacterial Activity of Produced Copper Carbonate Nanoparticles
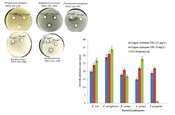
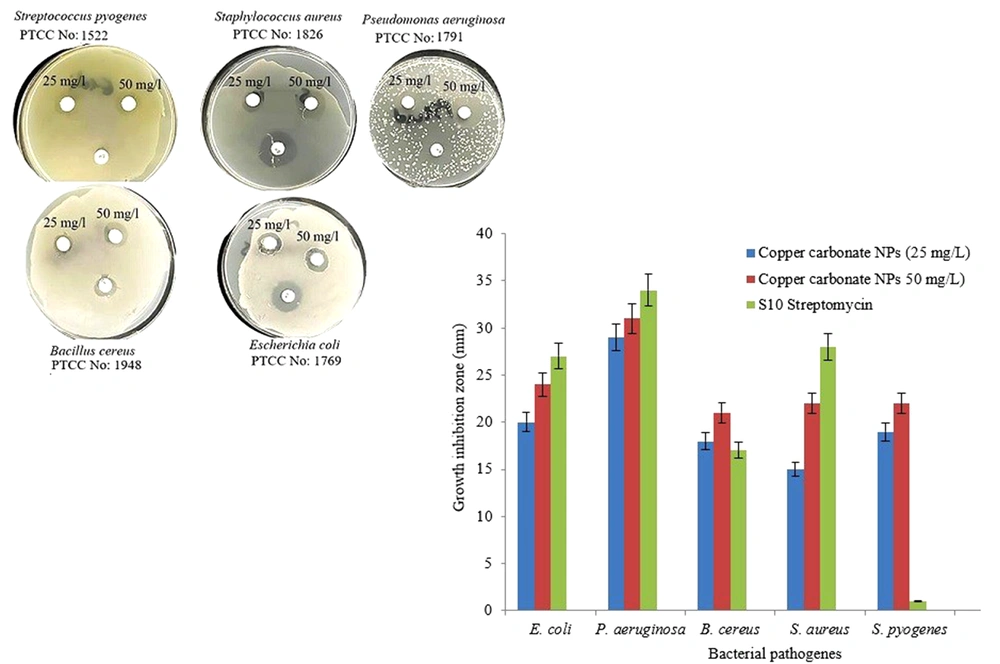
The disc diffusion test was employed to analyze the antibacterial activity of copper carbonate nanoparticles (17). The antibacterial activity of copper carbonate nanoparticles (25, 50 mg/L) was tested on the bacterial isolates, such as Escherichia coli PTCC1769, Staphylococcus aureus PTCC1826, Streptococcus pyogenes PTCC1522, Pseudomonas aeruginosa PTCC1791, and Bacillus cereus PTCC1948. For this experiment, 6-mm sterile paper discs were soaked in 20 µL of copper carbonate nanoparticle colloidal solution for 1 hour. Bacterial suspensions with a concentration of 0.5 McFarland (1.5 × 108 colony-forming unit [CFU]/mL) were cultured on Mueller-Hinton agar (MHA), and discs of paper were inserted to determine the inhibition of bacterial growth close to the disc. The inoculated Petri dishes were permitted to incubate at 37°C for 24 hours. Bacterial sensitivity was determined by measuring the diameter of the zone of inhibition around each disk. Disks with the classic antibiotic (streptomycin, 10 g/disk) served as a positive control.
4. Results
4.1. Enrichment and Isolation of Urea-Hydrolyzing Copper-Resistant Fungi
Eighteen copper-resistant aquatic fungal isolates (designated ccf1-ccf18) with distinct morphological and morphological traits were identified and purified using the enrichment culture technique on PDA agar culture media enriched with copper chloride at a final concentration of 25 mM. A qualitative assessment was conducted to investigate the ureolytic activity of the fungal isolates. The urease enzyme activity test was positive in 15 out of the 18 selected fungal isolates (Figure 1). Fungal isolate ccf7, however, showed the highest urease enzyme activity based on the color change of the urea agar-based culture medium and the incubation duration. The isolate was characterized as a superior isolate with significant urease activity since it was able to transform the color of the culture medium to brilliant pink after 24 hours of incubation.
4.2. SEM and DLS Analyses of Copper Carbonate Nanoparticles Produced by Fungal Isolate ccf7
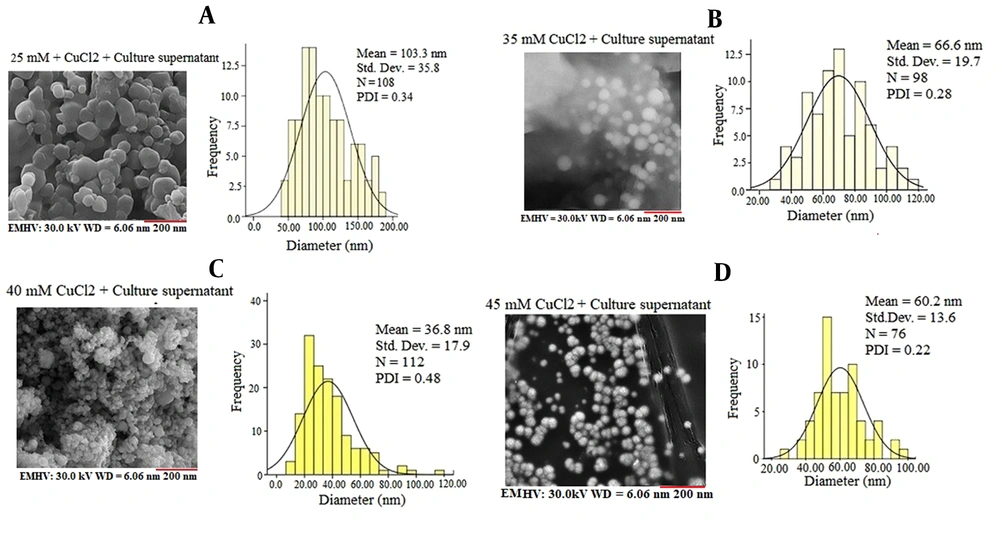
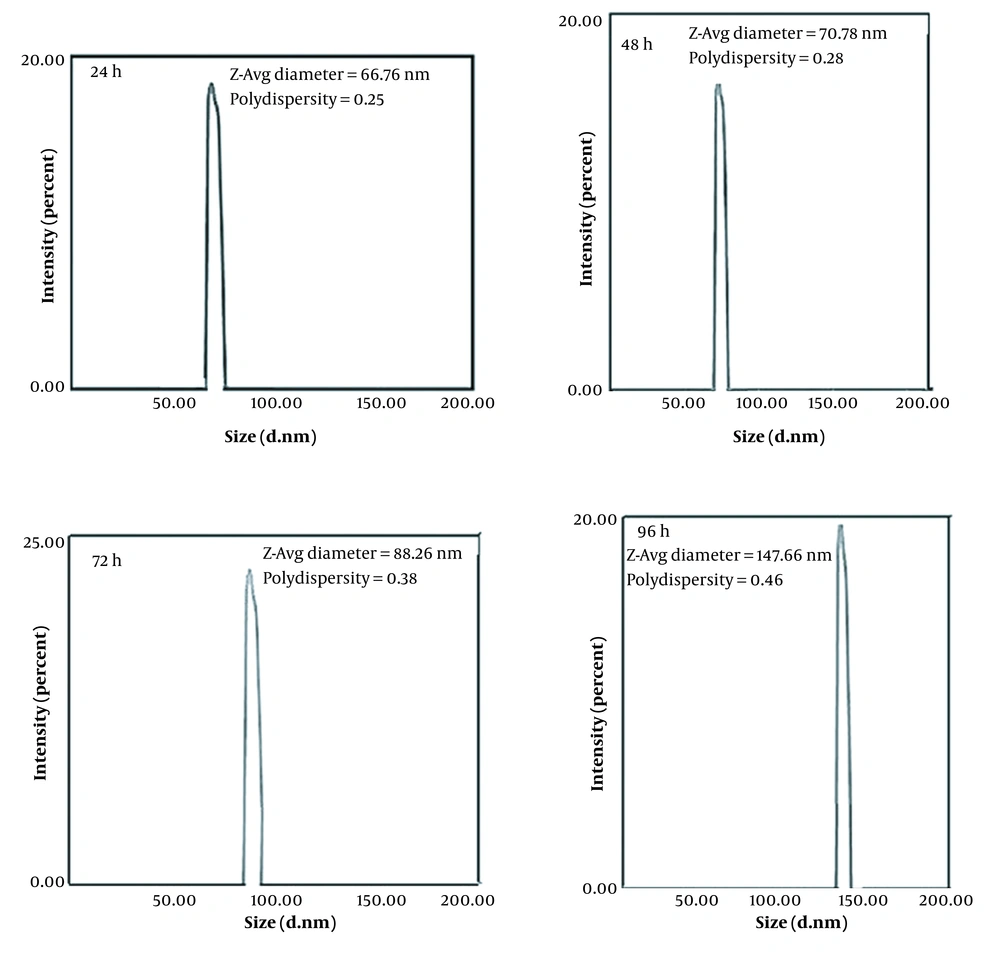
Based on the results of copper chloride salt tolerance and qualitative assessment of urea hydrolysis capability, fungal isolate ccf7 was examined by SEM and DLS techniques for copper carbonate nanoparticle production experiments in a filtrate free of mycelium. With different concentrations of copper chloride salt, the SEM images showed a relatively uniform arrangement of copper carbonate nanoparticles with globular shapes and an average particle size of 36 to 103 nm (Figure 2). Figure 2A shows the SEM images of copper carbonate nanoparticles synthesized with the CFE of fungal isolate ccf7 containing biocarbonate treated with copper chloride salt at a final concentration of 25 mM. As seen in Figure 2A, spherical- and ellipsoidal-shaped irregular nanoparticles were produced, with a mean diameter of 103.3 nm and a polydispersity index (PDI) of 0.34. An SEM analysis was used to explore the surface structure of copper carbonate nanoparticles formed by the CFE of fungal isolate ccf7 exposed to 35 mM copper chloride in the reaction mixture, showing the synthesis of regular spherical nanoparticles with a mean particle size of 66 nm and a PDI of 0.28 (Figure 2B). An analysis of the surface structure of copper carbonate nanoparticles created by CFE exposed to 40 mM copper chloride showed the production of spherical nanoparticles with an average diameter of 36 nm and a PDI of 0.48. (Figure 2C). Based on the FESEM examination, the CFE of fungal isolate ccf7 treated with copper chloride salts at a concentration of 45 mM generated copper carbonate nanoparticles with an average size of 60.2 nm and a PDI of 0.22 (Figure 2D). When the copper chloride concentration was lower, the nanoparticles were bigger, and the shapes were more irregular. Although the size of the nanoparticles in the copper chloride salt concentration of 40 mM significantly decreased when compared to other concentrations and had a more suitable size, the concentration of 45 mM can be considered optimal based on the monodispersity index, regular morphology, and lack of lumpiness of the particles. It was chosen as the optimal concentration for further testing. A DLS analysis was used to examine the effects of the incubation duration (24, 48, 72, and 96 hours) on nanoparticle size in the presence of CFE treated with 45 mM copper chloride stock (Figure 3). The DLS analysis revealed the size variation of copper carbonate nanoparticles generated in the reaction mixture at varied incubation durations in the range of 66 to 147 nm with DPIs ranging from 0.25 to 0.46. Based on the data, the average particle size in the first 24 hours of the reaction was calculated to be 66.7 nm, indicating that the produced nanoparticles have a reasonable distribution based on the PDI of 0.25. Meanwhile, increasing the incubation period up to 96 hours increased the size of the nanoparticles and decreased the monodispersity index; in other words, the initial 24 hours of the reaction is the best incubation time for the synthesis of copper carbonate nanoparticles.
Field emission scanning electron microscope images and histogram of the particle size distribution of copper carbonate nanoparticles generated by the mycelium-free extract of the selected fungal isolate ccf7 treated with varied concentrations of copper chloride at 25°C and a shaker speed of 100 rpm
The dynamic light scattering analysis results of copper carbonate nanoparticles generated in different durations of incubation in the reaction mixture by the mycelium-free extract of the selected fungal isolate ccf7 treated with 45 mM copper chloride at 25°C and a shaker speed of 100 rpm (Abbreviation: PDI, polydispersity index.)
4.3. The Results of the EDX and FTIR Study
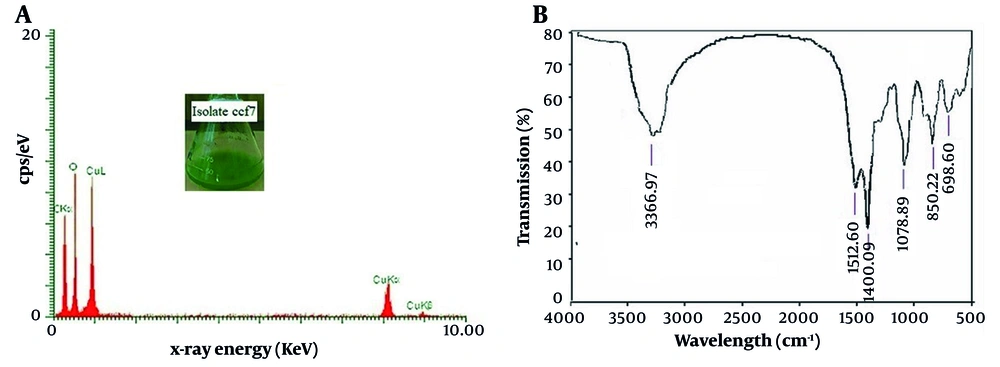
As illustrated in Figure 4A, the EDX analysis reveals a strong signal from copper atoms (1, 8, and 9 keV), oxygen (0.5 keV), and carbon (0.25 keV) atoms, indicating the presence of copper carbonate nanostructures (CuCO3(OH)x). The active presence of several functional groups on the surface of produced copper carbonate nanoparticles was revealed by the FTIR analysis (Figure 4B). According to the results of the FTIR analysis, the broad peak at 3366 cm-1 relates to the hydroxyl group in the aqueous solvent or to the hydroxyl group connecting with amino compounds as a reducing or capping agent involved in the formation of nanoparticles. The strong peak at a wavenumber of 1400 cm-1 is caused by the asymmetric stretching vibrations of the amid region with specific amino acids. The carbonate group is represented by the peak at 1512 cm-1 (CO3-2). Furthermore, according to credible sources, the peaks in the areas of 1078, 850, and 698 may be a reflection of the existence of carbonate ions (13, 14).
A, Energy-dispersive X-ray spectroscopy analysis and B, Fourier transform infrared spectroscopy analysis of copper carbonate nanoparticles formed in the reaction mixture containing the mycelium-free extract of the selected fungal isolate ccf7 treated with 45 mM copper chloride at 25°C, a shaker speed of 100 rpm, and then 24 hours of incubation
4.4. The Results of Phenotypic and Molecular Identification of the Selected Fungal Isolate ccf7
After 5 days of incubation at 25°C, the fungal colonies' growth rate was equivalent to 2.2 to 2.5 cm, which was visible as muddy green color. Conidia were ovoid to oval in shape and had transverse walls created in chains with branches on the conidiophore stalk. Then, using a set of ITS1/ITS4 primers, the ITS regions were amplified to determine the precise identity of fungal isolate ccf7. The BLAST results showed that the sequence of the extracted isolate had the highest similarity of 99% with the strains belonging to the genus Alternaria. This isolate was deposited in GenBank as Alternaria sp. strain ccf7 with accession number OP242500.
4.5. The Results of Antibacterial Effects
The biologically produced copper carbonate nanoparticles’ antibacterial activity against human pathogenic bacterial isolates at 2 concentrations of 25 and 50 mg/L and comparison with the antibiotic streptomycin showed that the nanoparticles had a good ability to inhibit the growth of the tested bacteria (Figure 5). According to the results, the maximum in vitro inhibitory effect of these nanoparticles against P. aeruginosa was 31 mm at 50 mg/L and 29 mm at 25 mg/L concentrations. The inhibitory effects of copper carbonate nanoparticles on P. aeruginosa and E. coli were more pronounced than on S. pyogenes, B. cereus, and S. aureus.
5. Discussion
Fungi have a high capacity for metal ion absorption and accumulation, which can lead to the formation of metal nanoparticles (10). According to the literature, ureolytic fungi are great candidates for metal carbonate synthesis. There have been reports of fungus creating metal carbonate. Li and Gadd (14) successfully synthesized copper bicarbonate by interacting with the supernatants of ureolytic fungus Pestalotiopsis sp., Myrothecium gramineum, and Neurospora crassa at optimal temperature, pH, and copper ion concentration. Using the supernatant of ureolytic fungal cultures, Liu et al. (13) investigated the relationship and involvement of fungal proteins in the formation of copper carbonate nanoparticles and concluded that triose phosphate isomerase (TPI) plays an important role in the synthesis and morphology of copper carbonate nanoparticles. In this study, the ability of the aquatic fungus Alternaria sp. strain ccf7 to create copper carbonate nanoparticles was investigated using a CFE approach. The findings demonstrated that Alternaria sp. strain ccf7 was capable of generating spherical copper carbonate nanoparticles within 24 hours after incubation at 25°C and a shaking rate of 100 rpm at an optimal concentration of 45 mM copper chloride. Extracellular synthesis of metal nanoparticles using the approach of CFE is preferable to other ways because it is faster and removes processes such as cell lysis and filtering in purification and extraction stages. On the other hand, because it does not require additional and complex procedures (such as the use of ultrasonic waves to prepare and perfect nanoparticles) and is less expensive, it will be more efficient for nanoparticle production (18, 19). The Alternaria fungus isolated in this study is one of the aquatic fungi. In recent years, aquatic fungi have been identified as prospective biofactories for the production of ecologically benign and low-cost metal nanoparticles (20). These fungi can accumulate and detoxify metals via various enzyme reduction pathways and transform metal salts into metal nanoparticles (21). Several aquatic fungi can generate inorganic crystals, nanostructures, and metal nanoparticles with chemical-like characteristics while maintaining perfect control over the particle size and shape (22). Alternaria sp. strain ccf7 was a urease-positive fungus that could adjust the pH of the medium and turn it pink. Urease (EC 3.5.1.5) is a nickel-containing enzyme that converts urea to ammonia and carbamate. Urease may be created in response to a microorganism’s stress to cope with the low pH of the environment (9). The morphology and size of biologically generated nanoparticles are affected by a variety of physical and chemical parameters, such as metal ion concentration, reaction temperature, incubation duration, and pH. The researchers have concluded that raising the precursor solution concentration promotes nanoparticle creation and affects nanoparticle morphology (23). Several concentrations of copper chloride salt were explored in this study to achieve the optimal concentration of copper chloride, and Alternaria sp. strain ccf7 showed the best result at a concentration of 45 mM. The incubation time has a considerable influence on the size, shape, and rate of synthesis of nanoparticles by microorganisms (24). We studied the influence of the incubation period at 24, 48, 72, and 96 hours. After 24 hours, the average size of the particles in the reaction was calculated to be 66.7 nm, indicating that the produced nanoparticles have an appropriate distribution based on the PDI of 0.25. Since the development of nanotechnology, it has been possible to use nanoparticles to overcome therapeutic constraints. Then, by lowering the dosage of medications, drug resistance, and its side effects can be decreased (25). Metal nanoparticles have attracted attention due to their wide range of applications and have opened up a new field in medical science. Nanotechnology has shown that increasing particle size from micrometers to nanometers improves reactivity and creates an antimicrobial effect. The bactericidal reaction of copper nanoparticles is initiated by releasing Cu2+ ions. These positively charged ions are absorbed by the carboxylic groups found in the lipoproteins of the bacterial cell wall, resulting in the formation of reactive oxygen species (ROS), which causes oxidative stress in the bacterial cell, enzyme dysfunction, DNA damage, and subsequently bacterial death (26). The antibacterial activities of copper carbonate nanoparticles formed by the CFE of Alternaria sp. strain ccf7 on human pathogens were examined in this research. According to the findings, the nanoparticles had a greater inhibitory impact on gram-negative bacteria (P. aeruginosa and E. coli) than on gram-positive bacteria (S. pyogenes, B. cereus, and S. aureus).
5.1. Conclusions
Copper carbonate nanoparticles have numerous advantages in a variety of industries, and they may be a viable alternative to gold and silver nanoparticles. These nanoparticles are created using various physical, chemical, and biological processes, with the biological synthesis approach being used due to the stability of the produced nanoparticles, high monodispersity, generation of non-toxic chemicals, and compatibility with the environment. Alternaria is a fungus genus with multiple species that are notable for their ability to create a wide range of secondary metabolites, including mycotoxins and pigments. According to some studies, Alternaria species have a high potential for nanoparticle synthesis, including silver, gold, and zinc oxide, due to their capacity to synthesize a diverse set of enzymes that can be used in a variety of applications. The capability of Alternaria sp. strain ccf7 for the extracellular manufacture of copper carbonate nanoparticles under the CFE technique was examined for the first time in this study. To produce copper carbonate nanoparticles, the copper chloride concentration and incubation period were optimized to acquire more homogenous and uniform nanoparticles with the desired particle size.