1. Background
Ovarian cancer is one of the most common women cancers worldwide that leads to most deaths among gynecologic malignancies. Although this disease's etiology is complex, genetic and environmental factors contribute to this complicated scenario (1, 2). Apoptosis, autophagy, and cell cycle are essential biological events with adjusted regulation in normal cells, which almost universally become disturbed in malignant and neoplastic cells. Recently, much attention has been focused on the prognostic role of apoptosis and cell cycle dysregulation in the onset of ovarian cancer (3, 4). More efforts have been directed toward investigating dietary supplements and other phytotherapeutic agents to increase efficacy and minimize the side effects of present chemotherapies (5). Silibinin, a natural polyphenolic flavonolignan isolated from milk thistle, has recently been known as a chemopreventive compound with amazing anti-cancer properties and fewer side effects than normal entities. Many studies have reported the inhibition of proliferation and cell cycle progression by silibinin in numerous cancers (6, 7). It has also been reported that silibinin alone can induce apoptosis and cell cycle arrest in the paclitaxel-resistant ovarian carcinoma cell line and sensitize these cells to paclitaxel (8-13). Silibinin is well-tolerated by the body and has proven less toxic than other anticancer therapeutics (7). Maleki et al. showed that silibinin inhibited cell growth and promoted apoptosis and reversion of the epithelial-mesenchymal transition in SKOV-3 and A2870 ovarian cancer cell lines (14). Another study reported that silibinin induced G2/M cell cycle arrest in Hela and SiHa cervical cancer cell lines via activation of the mitochondrial fission pathway (15). The present study aimed to clarify the effects of silibinin at various times and concentrations on the viability of a human ovarian cancer cell line of SKOV-3 and some of its underlying molecular mechanisms for silibinin-mediated cell cycle arrest and apoptosis in this cell line.
2. Objectives
Compared to similar studies, this study investigated the following issues: For the first time, we evaluated the silibinin effect on the cell cycle and some of its related gene expression. Moreover, this study showed the essential role of silibinin concentrations on its anticancer effects because silibinin administration in lower concentrations (12.5 µg/mL and 25 µg/mL) not only resulted in no significant decrease in cell viability but also slightly increased cell growth after 72 h.
3. Methods
3.1. Cell Culture
SKOV-3, a human ovarian cancer cell line, was obtained from the National Cell Bank of Iran (Tehran, Iran). We used RPMI-1640 (GIBCO, UK) medium (supplemented with fetal bovine serum (20%) and penicillin/streptomycin (1% v/v) (Gibco, Scotland) for culturing SKOV-3 cells at 37°C in 5% CO2 atmosphere under 90 - 95% humidity. Dimethylsulfoxide (DMSO, Sigma, USA) was the silibinin solvent.
3.2. Cell Viability Assay
The cell viability test was performed using 3-(4, 5 dimethylthiazol-2yl) 2, 5-diphenyl tetrazolium bromide (MTT, Sigma, USA) assay (16). Harvested cells were trypsinized using 0.25% trypsin) Sigma, USA), dyed with trypan blue, counted on a Neubar slide, and seeded into 96-well plates (8000 cells/well). The next day, different concentrations of silibinin (0, 12.5, 25, 50, 75, 100, 150, and 300 μg/mL) were administered to wells for 24, 48, and 72 hours. Each dose was tested on the six wells of the 96-well plates. Six cultured wells with DMSO incubation were used as the negative controls in each experiment. The amount of DMSO in all the experiments never exceeded 0.1% (v/v), and accordingly, the same amount of DMSO was added to control culture plates. At the end of the indicated incubation period, 10 μL of the MTT solution (5 mg/mL) was added to each well, followed by incubation at 37°C for 3h. After removing the culture medium, the insoluble formazan crystals, formed in living cells by the activity of mitochondrial dehydrogenases, were revealed by adding 100 μL of DMSO to each well. The absorbance values were measured at 570 nm using an ELISA reader (Awarnesse, USA). Individual samples were analyzed in quintuplets against a background of blank wells. Relative to the control experiment, the cell viability from three independent experiments (mean ± SD) was presented as the percentage of cell viability as follows:
3-(4, 5 dimethylthiazol-2yl) 2, 5 diphenyl tetrazolium bromide assay was carried out to investigate the cytotoxic effect of silibinin on SKOV-3 cells and sort out its appropriate Half maximal inhibitory concentration (IC50) value. IC50 was determined by probit analysis using the Pharm PCS (Pharmacologic Calculation System).
3.3. Flow Cytometry Analyses: Assessment of Apoptosis and Cell Cycle
Floating cells in medium and 106 harvested attached cells were collected after treatment with silibinin (50, 75, 100 μg/mL for 48 h) and washed twice with Phosphor buffered saline (PBS). Then, cells were double stained with annexin annexin V conjugated with fluorescein isothiocyante (V-FITC) and propidium iodide (PI) by Annexin V-FITC Apoptosis Detection Kit (Abcam, UK) and analyzed by flow cytometry (BD Biosciences, San Jose, CA, USA). All experiments were carried out in triplicate.
65×104 cells were seeded as described earlier to analyze the cell cycle distribution. On the next day, the cells were serum-starved for 24h before silibinin administration (50, 75, and 100 μg/mL for 48h). In short, floating cells in medium and harvested cells were washed twice with cold PBS and then fixed with cold 70% ethanol at 4°C. After 2h, the fixed cells were stained with 10 μL (2 μg/mL) PI (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) in the presence of 5 μL (100 μg/mL) of Ribonuclease (Sigma-Aldrich, St. Louis, US) for 30 min at 37°C. Finally, data obtained from a fluorescence-activated cell sorter were analyzed using Win MDI 2.9 software (Beckman Coulter, CA, USA).
3.4. Quantitative RT-PCR
Total RNA was extracted from SKOV-3 cells using the RNX Plus™ kit (CinnaClon, Tehran, Iran), according to the manufacturer’s instructions. The 260/280 absorbance ratios and RNA concentration measurements were performed using Nanodrop (Thermo Scientific, Wilmington, USA). The 260/280 OD ratio of all the samples was recorded between 1.8 and 2.2, indicating their high purity. The quality of the extracted RNA was determined by formaldehyde–agarose gel electrophoresis (1.2% agarose; Gibco/BRL). RNase-free DNase (TAKARA, Japan) treatment of the total RNA was performed to eliminate any potential traces of genomic DNA. Based on the supplier's protocol, the first-strand cDNA was synthesized from 2 µg of total RNA from each sample using the cDNA synthesis kit (RR037Q, Takara BIO INC). Gene expression was assessed by QRT-PCR for the genes of interest (B-cell lymphoma 2 (Bcl-2), cyclin E, and S-phase kinase-associated protein 2 (SKP2)) on a Light Cycler (Rotor-Gene™ 6000 Real-Time PCR System, Corbett Life Science) with RealQ PCR 2X Master Mix (A320799 Ampliqon, Denmark). GAPDH (Glyceraldehyde 3-phosphate dehydrogenase) and ACTB (β-actin) genes were used as housekeeping genes to normalize the transcript amount in each sample. All primers were purchased from Bonyakhteh Stem Cell Research Center, Tehran, Iran. The specificity of the PCR products was evaluated by verifying a single peak in melting curve analysis. All real-time PCR reactions were carried out in duplicate. The relative expression of the PCR products was measured using the 2-∆∆Ct method. Statistical analysis of primer efficiency was carried out by LinReg PCR Software (Amsterdam, The Netherlands, version 2012).
3.5. Statistical Analyses
The results were expressed as mean ± SD. All experiments were performed at least twice. Student’s two-tailed t-test and one-way variance analysis (ANOVA) were used to compare the data between two and more than two groups, respectively. A P-value < 0.05 was considered statistically significant. The data were graphically represented using SPSS 16.
4. Results
4.1. Inhibition of Cell Growth by Silibinin in a Dose- and Time-Dependent Manner
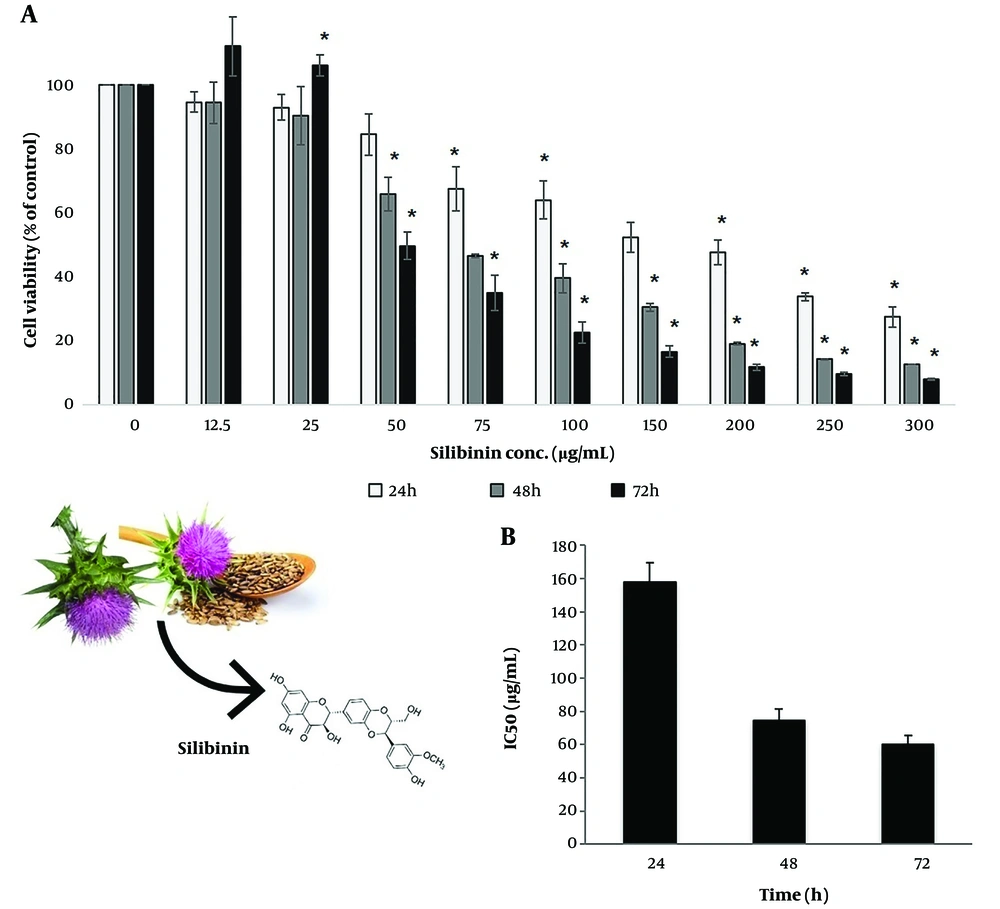
Different concentrations of silibinin (12.5 - 300 μg/mL) showed different cytotoxic effects against SKOV-3 cells for 24, 48, and 72 h. Silibinin treatment led to a concentration- and time-dependent decrease in cell viability. However, the lower doses of silibinin (12.5 μg/mL and 25 μg/mL) showed increased cell proliferation after 72 h. All doses in the 24 and 48 h and all doses in the 72 h, except for 12.5 μg/mL and 25μg/mL, significantly decreased the cell viability (P < 0.05; Figure 1). Figure 1 shows the IC50 of silibinin after 24, 48, and 72 h, which was estimated using the Pharm PCS statistical package (Springer Verlag, USA).
Assessment of silibinin effects on cell proliferation by 3-(4, 5 dimethylthiazol-2yl) 2, 5 diphenyl tetrazolium bromide (MTT) assay. (A) The SKOV-3 cells were treated with various concentrations of silibinin for 24, 48, and 72 hours, and their viability was assessed using an MTT assay. Results are presented as mean ± SD from at least three independent experiments (P < 0.05 by one-way variance analysis). (B) IC50 values during 24-, 48-, and 72-hours incubation times were investigated by MTT assays using the Pharm PCS (Pharmacologic Calculation System). Results are presented as mean ± SD from at least three independent experiments
4.2. Silibinin Induces Apoptotic and Cell Cycle Arrest in SKOV-3 Cells
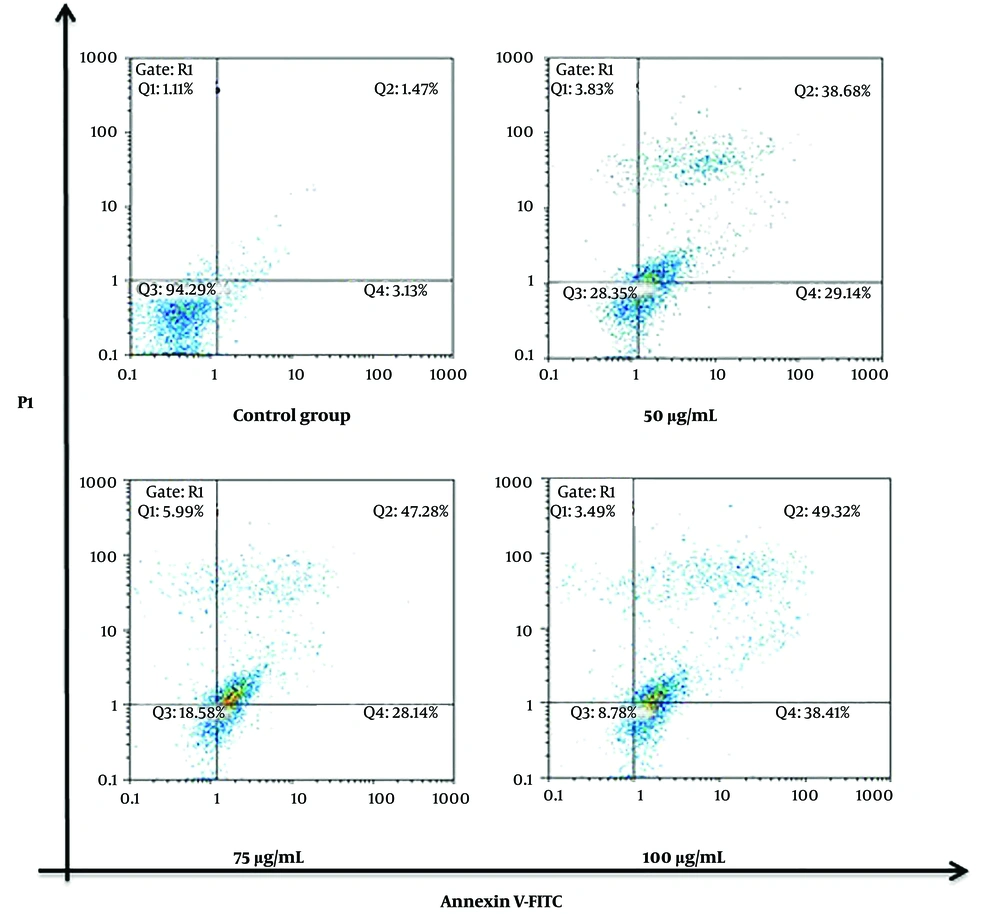
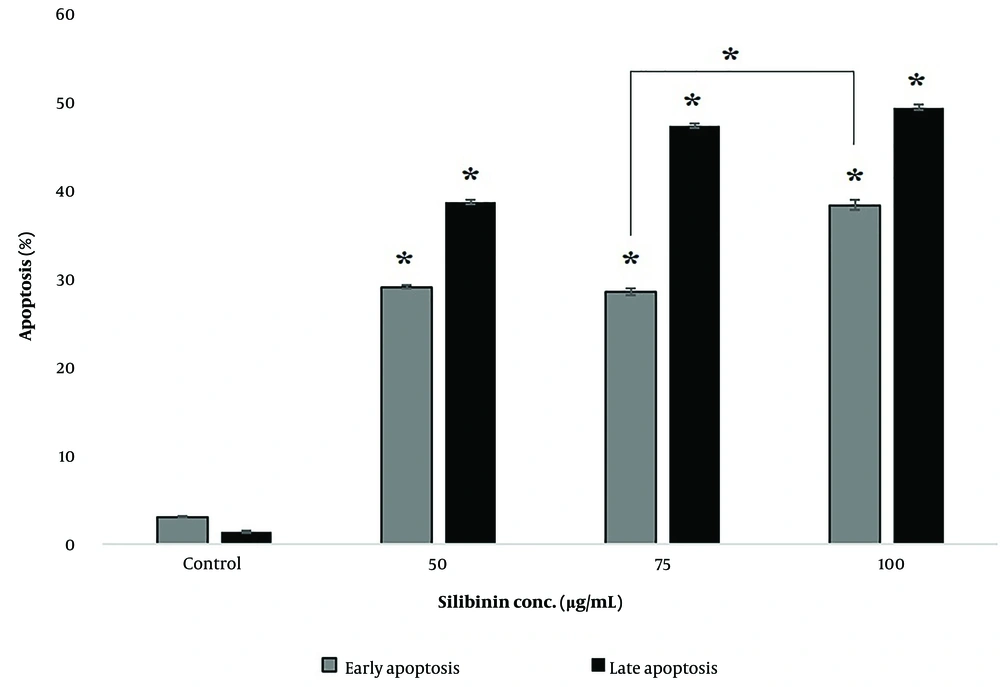
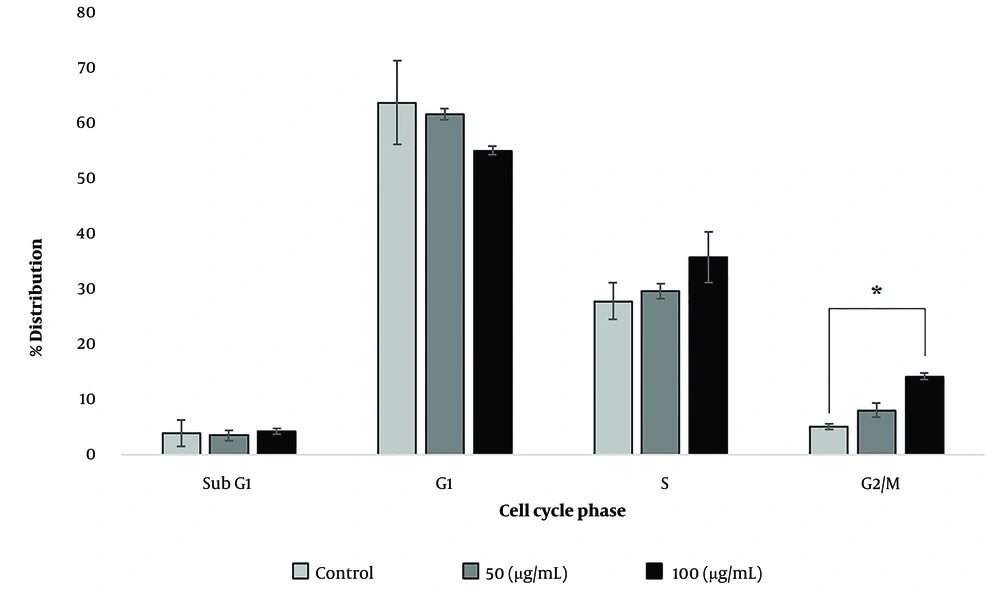
Flow cytometry analyses revealed the induction of enhanced apoptosis in the SKOV-3 cells treated with silibinin in a concentration-dependent manner after 48 h (Figures 2 and 3). These results were based on the onset of significant (P < 0.05) accumulation of late apoptosis (Annexin V+ and PI+: Q2 regions in Figure 2A). As shown in Figure 4, an accumulation of SKOV-3 cells was observed in the sub-G1 and G2/M phases after silibinin treatment. Notably, 100μg/mL silibinin caused a significant (P = 0.017) increase in the G2/M phase compared to the control. The proportion of cells in the G1 and S phases was markedly decreased in SKOV-3 cells after the administration of silibinin.
Silibinin-induced apoptosis in SKOV-3 cells. Cells treated with 50, 75, and 100 μg/mL of silibinin for 48 h were studied for apoptosis with Annexin V-FITC and PI. A scattered plot chart is drawn. Q1 region: Necrotic cells (Annexin V-/PI+), Q2 region: Late apoptotic cells (Annexin V+/PI+), Q3 region: Viable cells (Annexin V-/PI-), Q4 region: Early apoptotic cells (Annexin V+/PI-)
The percentage of early and late apoptosis in different groups after 48 hours. Data are statistically significant in all silibinin-treated groups (*P-value < 0.03) compared to control and also in 75µg/mL compared to 100µg/mL silibinin-treated groups of early apoptotic. Results are presented as mean (n = 3) ± SD. *, P < 0.05
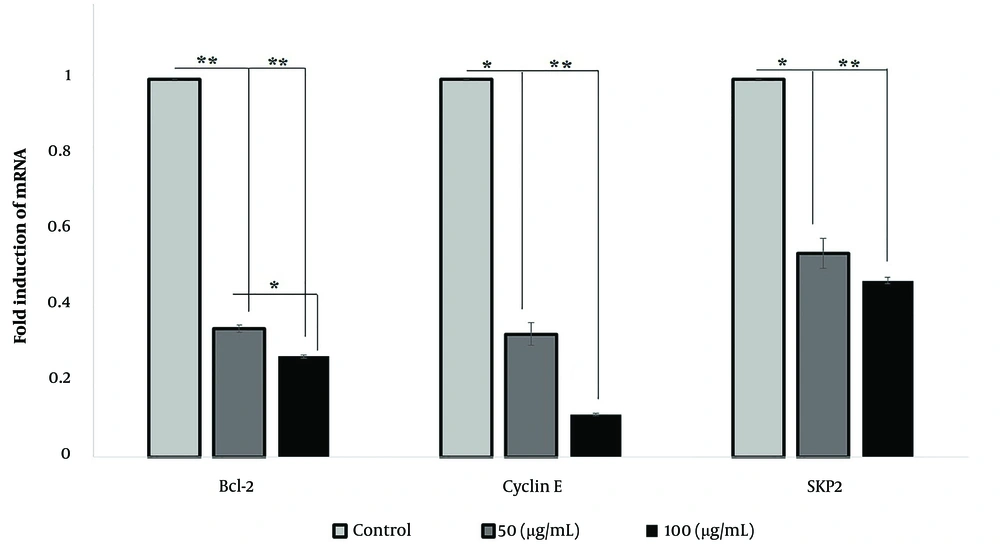
4.3. Investigation of Bcl-2, Cyclin E, and SKP2 Transcriptions Under Silibinin Treatment
We measured the expressions of Bcl-2, cyclin E, and SKP2 to determine their possible roles in the induction of apoptosis and cell cycle arrest after the administration of silibinin in SKOV-3 cells. The gene expression analyses indicated that two doses of silibinin significantly (P < 0.05) downregulated cyclin E, SKP2, and Bcl-2 (Figure 5).
5. Discussion
Silibinin can regulate various cell functions, including growth, proliferation, invasion inhibitory effects, cell cycle arrest, and apoptotic induction, which makes it suitable as an anticancer agent (6, 17). Some of silibinin's anticancer molecular mechanisms (e.g., down-regulation of Akt signaling pathway) against ovarian cancer have been studied previously (11, 18, 19). Furthermore, silibinin can enhance the anticancer activity of paclitaxel on ovarian carcinoma cell lines (12). Here, we studied the anticancer activities of silibinin against ovarian cancer SKOV-3 cell line by focusing on its cellular effects (such as apoptosis and cell cycle arrest). To achieve this, the effects of silibinin on viability, apoptosis induction, and cell cycle progression were explored in vitro. Notably, according to our previous reports (17), silibinin in high concentrations (from 100 to 300 µM) had no cytotoxic effect on the viability of MCF-10A cells as a non-cancerous human breast epithelial cell line. Therefore, silibinin can be a suitable candidate in clinical trials. According to the results of the present work, silibinin had dose- and time-dependent cytotoxic effects in SKOV-3 cells. Interestingly, the optical density of SKOV-3 cells in 12.5 µg/mL and 25 µg/mL doses of silibinin after 72 h was slightly increased. This increase can result from the excess of cell proliferation or metabolic activity of cells compared to controls and needs further experiments for confirmation. However, the concentration mentioned above of silibinin at 24 and 48 h induced negligible changes in cell viability. To explore the cytotoxic mechanism of silibinin in concentrations of more than 25µg/mL, we studied the apoptotic induction property of this compound against SKOV-3 cells. Silibinin significantly caused dose-dependent early apoptosis in treated SKOV-3 cells. Apoptosis is preferable to induced cell death by anticancer drugs because of its immune-quiescent characteristic (20). Bcl-2 is an anti-apoptotic factor overexpressed in many cancers (21), and silibinin decreased Bcl-2 mRNA levels. Apoptosis is revealed by discrete sub-G1 peaks on DNA content because DNA fragmentation is one of the main features of fractionated apoptotic cells or apoptotic bodies (22). In the present study, increased sub-G1 distribution represented the dose-dependent secreted apoptotic bodies from the cells (23). The apoptotic assay in Figures 2 and 3 represents this result, which represents dose-dependent dead cells.
Silibinin decreased cyclin E and SKP2 mRNA levels. Cyclin E has an important role in the progression of the G1 phase of the cell cycle (24), but we observed G2/M cell cycle arrest and not the G1 arrest here. SKP2 is one of the proteins in the Skp1-cullin 1-F-box (SCF) ubiquitin ligase complex, which acts as a substrate-binding arm. SCF ubiquitin ligase plays an important role in the progression of the G1 and S phases of the cell cycle. Upregulation of Skp2 is observed in numerous human cancers (25). However, alterations in cyclin E and SKP2 expression were insufficient for inducing the G1 arrest. One suggested reason for such contradictory effects is that these genes may have a functional mutation in the used cell lines, or other upstream or downstream genetic changes in SKOV-3 cells might have led to this outcome.
5.1. Conclusions
In summary, our findings indicated that the inhibitory effects of silibinin were mainly associated with the induction of apoptotic cell death and G2/M phase cell cycle arrest in SKOV-3 cells. In SKOV-3 cells in lower doses of silibinin, such as 50µg/mL, apoptotic death was induced. In cancer therapy research, triggering apoptosis at lower concentrations is favored to decrease the systemic toxicity of anticancer drugs. Silibinin can also overcome resistance mechanisms, reducing the effectiveness of conventional anticancer agents, and this necessitates evaluating the preventive and intervention strategies for silibinin in ovarian cancer pre-clinical models. Further research for elucidation of the underlying mechanisms of silibinin effects in ovarian cancer (in vitro and ex vivo studies) and for pre-clinical/clinical usage improvement (such as chemical modification for increased water solubility of silibinin) is warranted. Based on the evidence presented, silibinin appears to have the potential to be an effective chemopreventive agent for ovarian cancer.