1. Background
Large intestine cancer, also known as colon cancer or rectal cancer, is the growth of cancer cells in the colon or rectum. Among cancers, this cancer is the third most common cause of death, and among Iranian men and women, this cancer is ranked fourth and is the second most common cancer, respectively (1). This disease has the ability to spread to other parts of the body, such as the liver, kidneys, ovaries, and other abdominal organs. Some of the signs and symptoms of this disease include intestinal rupture and obstruction, abdominal pain, nausea and vomiting, the presence of blood in the stool, and constant fatigue. The risk factors that cause colon cancer are mainly caused by lifestyle and adverse environmental factors, such as improper diet, alcohol consumption, obesity, smoking, and lack of physical activity, and a small number of cases are caused by hereditary genetic factors (1-3).
One-third of cancers are curable if detected in the first stage. According to the complex nature of cancer and the involvement of several genes in the occurrence of such abnormality, there are different ways to treat it according to the type and degree of cancer progression. Common methods of cancer treatment, such as surgery, radiation therapy, and chemotherapy, and new methods of cancer treatment, which include gene therapy, immunotherapy, targeted therapy, alternative therapy, the use of cell therapy, hormone therapy, and nanotechnology, are used to treat this cancer (4, 5).
Nanoparticles (NPs) are used in nanotechnology. Nanoparticles contain particles in the size range of 1 to 100 nanometers. Nanoparticles can originate from natural or human sources. In recent years, we have witnessed unprecedented growth in the field of research and applications of nanotechnology, especially in the treatment of cancer (6). The aim of this study was to use multiple components, including drug-loaded NPs, to determine cytotoxicity and apoptotic gene expression in colon cancer cells.
2. Objectives
5-Fluorouracil or 5-FU (Adrucil) is a cytotoxic chemotherapy drug. It is used by intravenous injection to treat colon cancer, stomach cancer, esophageal cancer, pancreatic cancer, breast cancer, and cervical cancer. It causes cytotoxicity by interfering with essential biosynthetic activities by inhibiting the action of the thymidylate synthase enzyme or by miscombining its metabolites during ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) synthesis.
Among magnetic NPs that can be used as drug carriers in medical science, iron oxide NPs are the better choice due to the fact that transferring the drug to the targeted organ can decrease medication dosage and drug side effects (7). The purpose of this study was to investigate the anticancer effect of the combination of NP with 5-FU loaded on it on the cell survival of colon cancer cell line LS174T (8).
3. Methods
Scanning electron microscopy (SEM) image was captured by a TESCAN MIRA3 scanning electron microscope. X-ray diffraction (XRD) pattern was obtained with a Siemens diffractometer. Magnetization measurement was determined by MDKF vibrating sample magnetometer (VSM; Meghnatis Daghigh Kavir. Co., Kashan, Iran).
3.1. Synthesis of Fe3O4
The facile precipitation method was used for synthesizing metallic nanoparticles (MNPs). First, in the three-necked balloon, FeCl2 4H2O aqueous solution (10 mL, 0.3 M) was prepared. Then, the ferric chloride (10.0 mL, 0.6 M) was added to it at 80-85°C, and the ammonia solution (0.8 M) was added drop by drop. After finishing the droplets, the reaction was continued by magnetic decantation for 20 minutes. The black precipitate was dried in a vacuum oven after collecting and washing thoroughly with water. All of these steps were taken under an argon atmosphere.
3.2. Synthesis of Graphene Quantum Dots (GQDs)
In this study, 2 g of citric acid (CA) was heated up to 200°C by using a heating mantle in a 5 mL beaker. Then, after 35 minutes under vigorous stirring, the prepared orange liquid was neutralized via dropwise addition to the ethanolic NaOH solution with 10 mg/mL concentration. Finally, the synthesized graphene quantum dots (GQDs) were kept at 4°C and in a dark situation.
3.3. Preparation of Fe3O4/GQDs Nanohybrid
In this step, the mixture was refluxed under an argon atmosphere for 3 hours. By separating the precipitate with an external magnetic field, it was washed with ethanol several times. Finally, Fe3O4/GQDs nanohybrids were dried in a vacuum oven at 60°C.
3.4. Preparation of Cu-(BDC)/Fe3O4/GQDs Nanocomposite
In a 100 mL volume of N, N-dimethyl formamide, 1,4-benzenedicarboxylic acid (BDC; 1.00 mmol, 0.16 g), copper nitrate trihydrate (1.00 mmol, 0.24 g), and Fe3O4/GQDs (1.00 g) were combined and refluxed for 24 hours. The resulting precipitate was collected after the reaction cooled to ambient temperature by removing excess solvent through an external magnetic field. It was then washed three times with DMF and water. Finally, Cu(BDC)/Fe3O4/GQDs was dried at 50°C in a vacuum oven for 24 hours.
3.5. Drug Loading Studies
In 10 mL of 5-FU stock solution (1000 ppm), for the loading of 5-FU, 100 mg from Cu(BDC)/Fe3O4/GQDs nanocomposite was immersed and continuously shaken for 48 hours in the dark. The unloaded 5-FU drug was removed by washing quickly with distilled water and then dried vacuum at 45°C. The UV-vis technique was used for determining the loading efficiency of 5-FU at 266 nm (Figure 1).
3.6. Cell Culture
Colon cancer cell line LS174T was purchased from the Pasteur Institute of Iran. Moreover, RPMI culture media were used for culturing the cell, and it was kept in a freezer at -80°C. This cell line was derived from spindle type B colon epithelial cells of a 58-year-old woman, which became adherent after culturing.
The culture mediums that are used for cells consist of 2 parts: Basic environment and supplements. In this study, 45 mL RPMI 1640 basic culture medium was used from 10% (5 cc) fetal bovine serum (FBS), 1% (0.5 cc) streptomycin-penicillin antibiotic combination, 1% (0.5 cc) L-glutamine, and 1% (0.5 cc) mixture of non-essential amino acids. It was necessary to add these items to create 50 mL of complete culture medium. After cell culture, the cells were placed in an incubator with a temperature of 37ºC and 5% CO2.
3.7. MTT Test
In order to measure the toxicity of a chemical compound or any other substance on the cell, the MTT test or the toxicological test is used. Each well of the 96-well plates was filled with 100 μl of culture medium containing cells with a sampler (seeding). Then, the cells were incubated overnight (24 hours in total), and the next day, the culture medium was changed. Then, according to the previous design of the plate, different doses of 4 compounds, including control, empty drug, empty NP, and the combination of NP loaded on it (0, 0.1, 1, and 2), were added to the cells into each well. Then, after incubation for 24 hours, the contents of each well were poured out, and 75 μL of MTT was poured into each well. Then, the plate was placed in the incubator for 4 - 5 hours. After 4 - 5 hours, the wells were washed with 50 - 100 µL phosphate-buffered saline (PBS), and then 100 μL of isopropanol was added to each well with a Sampler. After incubating for 15 minutes, it was taken to read the number of each well, and optical density was rated at 560 nm. The measurement of MTT in 48 and 72 hours was carried out in the same way in the following days. Finally, the light absorption of each well was read by an enzyme-linked immunosorbent assay (ELISA) reader at a wavelength of 570 nm.
3.8. mRNA Extraction
Ribonucleic acid extraction from the cell was performed according to the instructions given in the ZiAzol Kit purchased from the ZiAViZ Company (ZiAViZTabriz, Iran). Then, the microtube containing messenger RNA (mRNA) was stored in the freezer.
3.9. Reverse Transcription Polymerase Chain Reaction
In order to evaluate the expression of genes in real-time polymerase chain reaction (PCR), mRNA must be converted to complementary DNA (cDNA) with reverse transcription polymerase chain reaction (RT-PCR). The PCR master mix preparation and other steps were continued according to the instructions in the Yekta Tajhiz Kit (Yekta Tajhiz, Iran).
3.10. Real-Time Polymerase Chain Reaction
The expression of BAX, BAD, and BCL-2 genes was assessed using quantitative polymerase chain reaction (qPCR). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was chosen as a reference gene. The sequence of the primers is shown in Table 1. In each reaction microtube, 0.5 μL cDNA (master mix), 10 μL SYBR Green, 2.5 μL RNA, 1 μL from primers, and 6 μL H2O were added equally.
| Primer Sequences | Primer Type | Gene |
|---|---|---|
| 5'-GGCCCACCAGCTCTGAGCAGA-3' | Forward | BAX |
| 5'-GCCACGTGGGCGGTCCCAAAGT-3' | Reverse | |
| 5'-GTGGAGGAGCTCTTCAGGGA-3' | Forward | BCL-2 |
| 5'-AGGCACCCAGGGTGATGCAA-3' | Reverse | |
| 5'-CAGTGATCTGCTCCACATTC-3' | Forward | BAD |
| 5'-TCCAGCTAGGATGATAGGAC-3' | Reverse | |
| 5'-GAGTCAACGGATTTGGTCGT-3' | Forward | GAPDH |
| 5'-GGTGCCATGGAATTTGCCAT-3' | Reverse |
Primer Sequences Used in Quantitative Polymerase Chain Reaction (qPCR)
The 4 steps of real-time PCR are set as follows respectively: (1) Denaturation: 95ºC for 2 minutes; (2) Denaturation (set for each cycle): 95ºC for 15 seconds; (3) Annealing: 60ºC for 15 seconds; (4) Synthesizing: 72ºC for 20 seconds.
3.11. Statistical Analysis
GraphPad Prism 9 (Graph Pad Software Inc., USA) was used to draw graphs and analyze the data.
4. Results
4.1. Characterization of Synthesized Nanocomposite
The morphological structure of the Cu(BDC)/Fe3O4/GQDs nanocomposites was investigated using SEM analysis, and the results are shown in Figure 1. The nanocomposites exhibit cubic crystalline frameworks of Cu(BDC) with spherical particles related to the presence of Fe3O4/GQDs nanohybrids (8, 9).
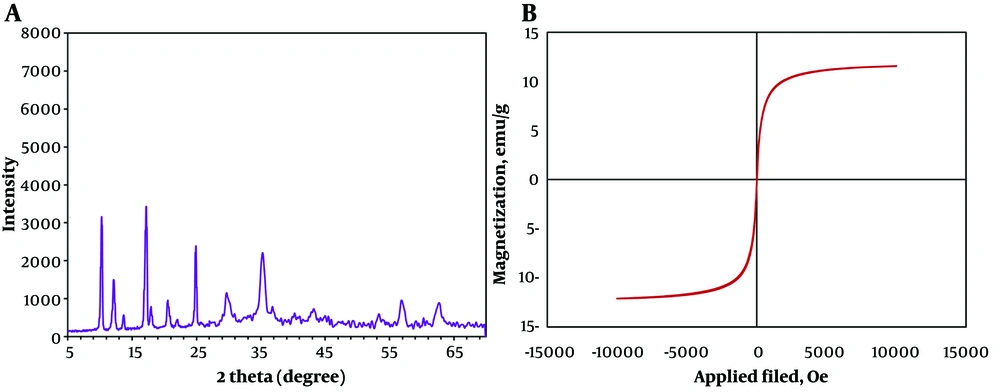
The crystalline structure of Cu(BDC)/Fe3O4/GQDs was examined through powder XRD analysis (Figure 2A). The typical diffraction of Fe3O4 NPs was observed at 2θ = 30.5°, 35.3°, 43.1°, 53.7°, 57.2°, and 62.8° in the XRD pattern of Cu(BDC)/Fe3O4/GQDs, corresponding to the (220), (311), (400), (442), (511), and (440) Bragg reflections, respectively (10). This demonstrated the existence of Fe3O4 NPs in the structure nanocomposite. Additionally, it showed characteristic diffractions at 2θ = 10.4°, 12.3°, 13.8°, 17.43°, and 24.8°, which can be attributed to the typical diffraction peaks of Cu-based Metal-Organic Framework (Cu-MOF) (8), thereby confirming that Cu-MOF was synthesized in situ in the presence of Fe3O4/GQDs.
The magnetization curve of Cu(BDC)/Fe3O4/GQDs was obtained using the VSM technique at room temperature (Figure 2B). It showed an S-like curve with about 11.5 emu/g magnetization. This amount of magnetization could transfer drug nanocarrier to a target site using an external magnetic field, which is beneficial in biomedical sciences (11).
According to a reported method (12), 5-FU was loaded into the Cu(BDC)/Fe3O4/GQDs nanocomposite by immersing it into the drug solution. The loading capacity of 5-FU was measured to be 90.3 ± 0.5% using UV-vis spectroscopy at 266 nm. It is noteworthy that the presence of Cu-BDC in the structure of nanocomposite significantly increased the loading capacity, which could be due to the possible interactions, such as host-guest interaction, electrostatic interaction, and hydrogen bonding with the drug (13).
4.2. Cytotoxicity Study Results by MTT Assay
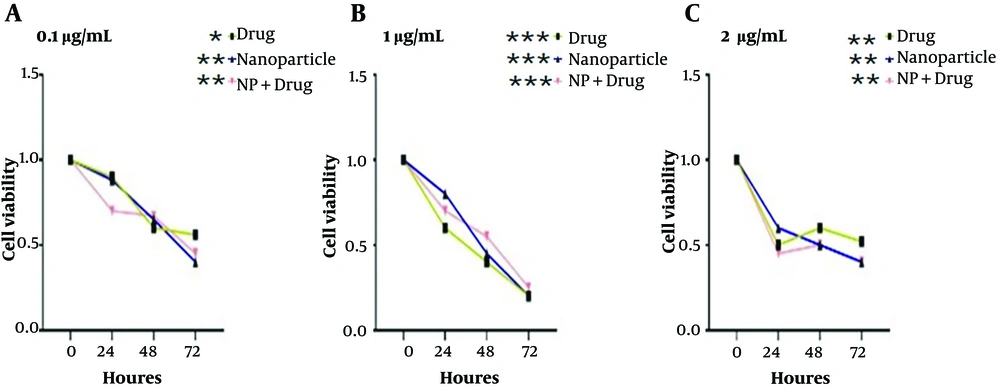
Cytotoxicity results are shown in Figure 3. Analysis with GraphPad Software version 9.0 showed that with increasing the concentration of drug loaded on the NP after 72 hours, the effect of toxicity increased, compared to the 2 forms of empty drug and empty NP.
4.3. Quantitative Polymerase Chain Reaction Results
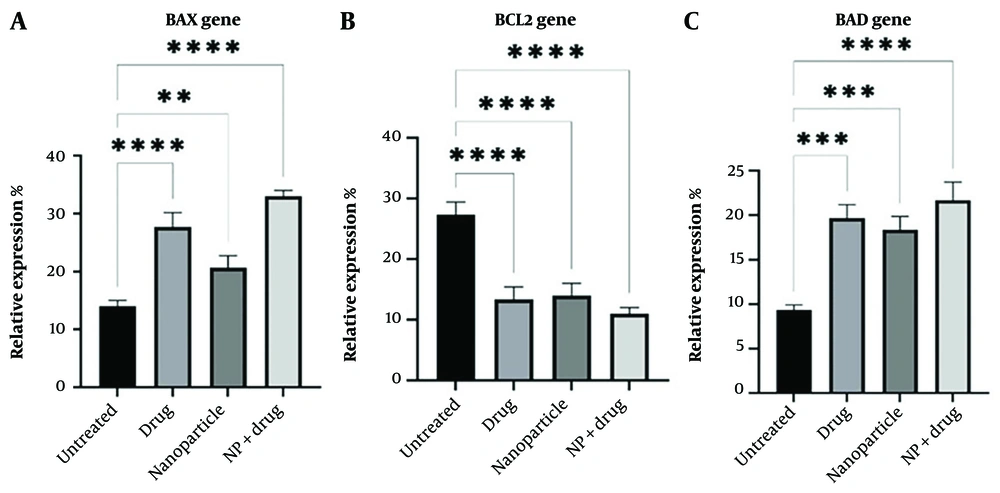
In general, the results showed the induction of apoptosis by nano-drug through increasing the expression of BAX and BAD and decreasing the expression of the BCL-2 gene (Figure 4).
5. Discussion
For investigating the present study’s hypothesis, MTT assay and qPCR were utilized. The results of the MTT test showed that at a concentration of 0.1 μg/mL, the combination of the NP with drug (NP+Drug) reduced the viability of colon cancer cell line LS174T in a dose and time-dependent manner, better than empty NP and empty drug, and this effect increased with the passing of time; accordingly, the survival rate of cells decreased in 72 hours, compared to 48 and 24 hours. Additionally, at a concentration of 1 μg/mL, the empty drug worked better than the combination of NP with the drug and empty NP, and this effect increased with the passing of time.
Furthermore, at a concentration of 2 μg/mL, the combination of NP with the drug showed better efficiency than the other two combinations; accordingly, this effect increased with the passage of time. Therefore, it can be concluded that the use of NPs as a carrier of 5-FU not only increases its solubility but also increases its absorption power, cytotoxicity, effectiveness, and the possibility of magnetic conduction to the desired target organ.
To determine the type of toxicity applied to LS174T colon cells, the expression levels of BAX, BCL-2, and BAD genes were studied in treated and untreated cells. Regarding the pro-apoptotic gene BAX, the drug-loaded on the NP worked successfully, and by increasing the expression of this pro-apoptotic gene, it caused the death of colon cancer cells. In the next step, the effectiveness of drug, NP, and control was higher. In the case of the anti-apoptotic gene BCL-2, the drug-loaded on the NP worked successfully, and by decreasing the expression of this anti-apoptotic gene, it caused the death of colon cancer cells. In the next step, the effectiveness of drug, NP, and control was higher. Regarding the pro-apoptotic gene BAD, the drug-loaded on the NP worked successfully, and by increasing the expression of this pro-apoptotic gene, it caused the death of colon cancer cells. In the next step, the effectiveness of drug, NP, and control was higher.
Previous studies’ findings confirm the current studies’ findings, some of which are as follows:
- An article presented by Amini-Fazl et al. showed that 5-FU drug was loaded on chitosan/poly acrylic acid/Fe3O4 magnetic nanocomposite hydrogel and could significantly reduce the viability and proliferation of cancer cells (14).
- An article presented by Ahmad et al. showed that ellagic acid (EA), sunitinib (STB), 5-FU, and cisplatin were loaded on chitosan-based hydrogel nanocarriers and could significantly reduce the viability and proliferation of cancer cells (15).
- Moreover, in other similar studies, 5-FU and other similar anticancer drugs were loaded on NPs containing different compounds, and then the effects of reducing the survival and cell proliferation of different types of cancer cells were proven (16, 17).
5.1. Conclusions
One of the important drugs used in chemotherapy to treat colon cancer patients is 5-FU. In the last few decades, by using nanotechnology to load the drug on the NP, the efficiency of the drug has been increased. For this purpose, magnetic iron NPs were used to increase the solubility, absorption, and cytotoxicity because the drug-loaded on NPs can accumulate in the target tissue under the influence of a magnetic field and be released from the NP under controllable conditions (9, 18). For this purpose, magnetic iron NPs were made, and then the drug was loaded on them. Then, the methods were used to check the size and shape of NP as a drug carrier, the amount of drug loading in the drug carrier, and, finally, the release rate of the drug.
Then, the MTT test was used to check the effectiveness of the nano-drug combination in preventing the growth of LS174T colon cancer cells in the in vitro environment. This study used combinations of control, empty NP, and empty drug with different concentrations, in addition to the combination of nanomedicine, for testing and investigation. Moreover, after the statistical analysis, it was concluded that, in general, the use of NPs causes severe toxicity compared to the two forms of empty drug and empty NP. In addition, the nano-drug induces cell apoptosis by increasing the expression of the pro-apoptotic genes BAX and BAD and decreasing the expression of the anti-apoptotic gene BCL-2.
The results of the current study confirm the previous findings in line with the therapeutic properties, including the anticancer properties of the 5-FU drug and the combination of this drug with NPs; in other words, the use of nano-drugs has great efficiency and capacity in the treatment of colon cancer that can be considered an important medicinal factor, along with other treatment methods.
Considering all the results, it can be concluded that the drug-loaded on NP can have anti-proliferative and pro-apoptotic properties. Further investigations can look deeper into this subject to determine the following issues: (1) Investigating the apoptotic effect of the present compound on other cancer cell lines; (2) evaluation of the therapeutic effects of 5-FU drug loaded on NPs in vivo; (3) necessity of studying other molecular pathways that are likely to be directly or indirectly affected by this compound; (4) studying the effect of this combination along with other chemotherapy drugs; (5) investigating the effect of this compound on normal human cells.