1. Background
Thyroid cancer is a prevalent endocrine cancer in the Iranian population, accounting for 3.5% of all cases (1). In recent years, the incidence of thyroid cancer has risen more rapidly than other malignancies, affecting both sexes and all races (2). Papillary thyroid carcinoma (PTC), a well-differentiated tumor, is the most common type of thyroid cancer, accounting for 58 to 85.9% of cases in different populations (3). PTC is more prevalent in regions with better diets and higher iodine intakes than other parts worldwide, while X-ray exposure is also a risk factor (4, 5). PTC tends to infiltrate neighboring tissues but does not metastasize distantly, resulting in an 87% survival rate after ten years (6). Papillary thyroid carcinoma risk factors include body mass index, metabolic syndromes, environmental pollutants, and a family history of thyroid nodules (7, 8).
Genetic changes are the most common cause of many benign and malignant growths, including thyroid tumors. However, mutations and chromosomal changes are less prevalent in PTC than in other thyroid tumors. The most frequent mutation observed in PTC is BRAF V600E (OMIM:164757), which disrupts the mitogen-dependent protein kinase (MAPK) pathway (9). Furthermore, patients with PTC may have simultaneous mutations in multiple members of the MAPK pathway, such as BRAF and RAS (OMIM: 190070) genes (10). Rearranged during transfection-PTC (RET-PTC) translocations and rearrangements of RET/NTRK3, RET/NTRK1, and RET/ALK have been observed in some PTC tumors (11). Gene expression changes are also a significant contributor to many cancers, although the gene expression profile in malignant PTC and benign goiter remains incompletely understood.
The gene known as RNA transcription, translation, and transport factor (RTRAF, ENSG00000087302) is located at chromosomal position 14q22.1. This gene exhibits a wide range of functionalities due to its presence in both the nucleus and the cytoplasm. In addition, RTRAF, a transcription factor, exhibits multifunctionality as a protein transporter and exerts significant influence over RNA destiny, centrosome architecture, and, above all, cell cycle progression (12). Empirical evidence suggests a possible association between RTRAF and influenza infection, germinoma of the central nervous system, and the development of specific malignancies, including brain tumors and colorectal cancer (13, 14). To the best of the authors' knowledge, whether this particular gene plays a role in the onset of thyroid cancer remains undetermined. Most thyroid tumors are identified through fine needle aspiration (FNA) and immunohistochemistry, which are both time-consuming and expensive and have the potential for error. In contrast, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) has emerged as a viable option to gauge gene expression in tumor cells and their surrounding normal tissues. This technique boasts about its low cost, high rate of efficiency, increased accuracy, and great affordability (15). Therefore, RT-qPCR can potentially serve as a valuable tool for identifying and assessing novel diagnostic molecular markers.
2. Objectives
The present investigation compared RTRAF gene expression levels between malignant thyroid tumors and adjacent healthy tissues, as well as benign thyroid tumors and adjacent healthy tissues. Notably, our findings revealed a significant reduction in the RTRAF gene expression in benign goiter tissues, while no significant alteration in the RTRAF gene expression was observed in malignant PTC tumors.
3. Methods
3.1. Sampling
We obtained informed consent from patients undergoing thyroidectomy at Sina Hospital in Isfahan. In accordance with the Helsinki protocol (IR.UI.REC.1398.058), 22 fresh PTC tissues and an equal number of healthy tissues adjacent to the tumor were collected for analysis. We also collected 10 fresh benign goiter tissues and 10 adjacent healthy tissues. Approximately 50 milligrams of fresh tissue were submerged into a stabilizing solution of RNAlater (Ambion Life Science Company, USA) according to the manufacturer's instructions. A 24-hour incubation was held at 4°C. Tissues were stored in a freezer at -70°C. Tissue samples with high blood content, high fat, unclear histological diagnosis, and small size were excluded from the study. Additionally, tumor samples lacking adjacent healthy tissues were not included in the study.
3.2. RNA Extraction
A tissue sample of 50 ± 5 mg was treated with RNAlater and washed with 0.9% saline. Liquid nitrogen was used to crush tissue, followed by RNA extraction using a Biobasic kit (Canada, Bio Basiclot: BS410A-N116DR0J) in accordance with the manufacturer's instructions. The quality of the extracted RNAs was evaluated using a 2% agarose gel. The concentration and purity of the extracted RNA were determined using a spectrophotometer (Nanodrop model OneC-USA), with samples containing low concentrations or contamination being excluded from the study.
3.3. Complementary DNA Synthesis
Using deoxyribonuclease I (DNase I) enzyme treatment (USA, Thermofisher, Lot: 00645766), genomic DNA was removed according to the manufacturer's instructions. Simultaneous use of thermal shock and ethylene diamine tetraacetic acid (EDTA) chelating agent was employed to terminate the enzyme in the reaction. In addition, cDNA synthesis was conducted using random hexamers.
3.4. Primer Designing and Selection of Calibrating Genes
Beacon Designer 8 software (Premier Biosoft, USA) was used for designing exon-junction primers. Evaluation of primer dimer and additional loops was accomplished with Oligo7 software (Molecular Biology Insights, USA). Table 1 presents primer specifications used in the study. The symplekin scaffold protein (SYMPK) gene was considered the reference gene (16).
| Gene (Accession Number) | Beacon Designer Scores | Oligo7 Scores | Sequence | Tm (°C) | PCR Product Length (bp) |
|---|---|---|---|---|---|
| RTRAF (NM_016039.3) | 59.8 | 894 | F5'GGTTTTGACACAGGAGATGC3' | 60 | 115 |
| R5'AACAGCTACTATGGCTTCGTTG3' | |||||
| SYMPK (NM_004819.3) | 82.6 | 729 | F5'ACG GTGCTGAGGGTCATTGA3' | 60 | 146 |
| R5'GAGGGTGGGACTTTGTCTGTGA3' | |||||
| GAPDH (NM_001256799.3) | 82.5 | 769 | F5'CCACTCCTCCACCTTTGACG3' | 58 | 107 |
| R5'CCACCACCCTGTTGCTGTAG3' |
Sequence and Characteristics of the Primers a
3.5. Reverse Transcription Quantitative Polymerase Chain Reaction
The RT-qPCR was conducted using a Bio-Rad chromo4 device (Bio-Rad-USA) in triplicate. The process included initial denaturation and enzyme activation at 95°C for 15 minutes. Afterward, 40 cycles were performed, including 30 seconds at 95°C, 30 seconds at primer connection temperature, and 30 seconds at 72°C, followed by the reading of the wells after each cycle. A negative control sample was used for each round of RT-qPCR.
3.6. Melting Curve Analysis
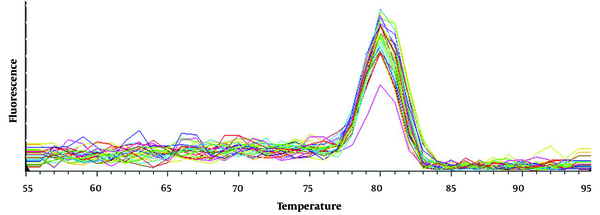
During the final RT-qPCR cycle, a melting curve was generated by gradually increasing the temperature from 55 to 95°C. The fluorescent changes in the green channel were then measured and recorded after each one-degree increase. Subsequently, we plotted the temperature on the X-axis and the derivative of the fluorescent changes on the Y-axis.
3.7. Statistics
The mean RTRAF gene expression was compared between goiter/PTC tissues and their adjacent healthy tissues using the Student's t-test. A relative expression analysis was conducted using REST 2009 software. The normality of continuous variables was assessed using the Shapiro-Wilks test, while Pearson’s correlation was used to determine the strength and direction of the relationship between the two variables. Significance was established with P-values below 0.05.
4. Results
4.1. RNA Quality and Quantity Analysis
The observation of ribosomal RNA bands on a 2% agarose gel suggests that the RNA remained undamaged during extraction (Figure 1). The intact RNAs had acceptable 260:280 and 260:230 absorption ratios ranging from 1.8 to 2.2 (data not shown). Samples lacking ribosomal RNA or with unacceptable absorption ratios were excluded from the study.
4.2. Melting Curve Analysis of Reverse Transcription Quantitative Polymerase Chain Reaction Products
A distinct peak was observed at 80±1°C, which aligns with the temperature forecast generated by the bioinformatics technique (Figure 2). The RT-qPCR selectively amplified the RTRAF gene, as evidenced by the sole peak. The lack of peaks at other temperatures indicates the absence of non-specific products and primer pairs.
4.3. Reverse Transcription Quantitative Polymerase Chain Reaction Data Analysis
The mean expression levels of all tissues in benign and malignant groups were compared to their adjacent normal tissues using REST 2009 software (Table 2). The expression of the RTRAF gene in benign goiter tissues was significantly reduced by five times compared to adjacent healthy tissues (relative expression = 0.154, p = 0.002). There was no significant difference in the RTRAF gene expression between PTC tissue and the healthy tissue adjacent to the tumor (relative expression =0.874, P = 0.808).
| Gene | Tissue | Expression | 95% CI | P-Value | Result |
|---|---|---|---|---|---|
| RTRAF/SYMPK | Benign goiter/ adjacent normal | 0.154 | 0.001 - 13.935 | 0.002 | Downregulation |
| Malignant PTC/ adjacent normal | 0.874 | 0.006 - 97.057 | 0.808 | NS |
Comparison of the RNA Transcription, Translation, and Transport Factor Gene Expression Relative to the Reference Gene in Benign and Malignant Thyroid Tumors Compared to Healthy Tissues Adjacent to the Tumor
4.4. Correlations between RNA Transcription, Translation, and Transport Factor Gene Expression, Tumor Size, and Patient Age
The RTRAF gene expression levels were compared among patients with varying tumor sizes and those with distinct age groups (Table 3). The RTRAF gene expression level was not associated with PTC tumor size (P = 0.1329) or patient age (P = 0.5815). Additionally, no correlation was found between PTC tumor size and patient age (P = 0.2022).
| Comparison | Pearson | 95% CI | P-Value | Result |
|---|---|---|---|---|
| RTRAF expression vs. tumor size | -1.574 | -0.6849 - 0.1119 | 0.1329 | NS |
| RTRAF expression vs. age | 0.56139 | -0.3305 - 0.5422 | 0.5815 | NS |
| Tumor size vs. age | -1.3237 | -0.6541 - 0.1666 | 0.2022 | NS |
Analysis of the Correlations Between the RNA Transcription, Translation, and Transport Factor Gene Expression, Tumor Size, and Patient Age a
5. Discussion
Understanding thyroid neoplasm behavior is important for disease prevention and treatment. Cancer development is linked to specific genes and changes in gene expression. Dysregulation of the cell cycle is a fundamental process in cancer development. Research has explored protein roles in cell cycle control, including investigating P27 protein levels in thyroid cancer (17). The expression of P27 was significantly higher in papillary hyperplasia of Graves' disease, indicating its usefulness in disease diagnosis. An immunohistochemistry analysis revealed that the expression of P27 was comparatively lower in papillary microcarcinomas (PMCs), which were metastatic in nature than their non-metastatic counterparts (P = 0.001), indicating that the gene mentioned above has the potential to serve as a diagnostic molecule for predicting the extent of invasiveness (18). Furthermore, the diminished expression of P27 exerts a more significant impact on the development of thyroid cancer compared to the excessive expression of cyclin D1 and cyclin E. Furthermore, immunostaining for P27 could potentially facilitate the identification of follicular variants of PTC, contributing to the disease diagnosis (19). Corroborating evidence suggests that cyclin D1 significantly contributes to the advancement of thyroid cancer (20). It has been suggested that the primary source of the transformation of differentiated PTC into incurable anaplastic thyroid carcinoma (ATC) is attributed to the abundance of cyclin D1 protein in ATC tissues. This increase in cyclin D1 protein levels is notably higher than in PTC tissues (20). The RTRAF gene is an indispensable factor in the advancement of the cellular replication process and the transition from the G1 to the S phase in breast cancer cells (21, 22). The RTRAF gene encodes a protein that stimulates cell cycle progression by regulating the expression of retinoblastoma (RB) and cyclin D1. Consequently, RTRAF has been suggested as a promising novel cell cycle regulator to serve as a biomarker for bladder cancer prognosis (22). The upregulation of the gene in question within bladder cancer tissues has been correlated with the presence of larger tumors (P = 0.001), lymph node involvement (P < 0.001), histological differentiation (P < 0.001), and poor survival. Additionally, the RTRAF gene overexpression in human nasopharyngeal and cervical carcinoma cells and tissues has been linked to suboptimal patient outcomes, larger tumor sizes, metastasis to lymph nodes, decreased survival rates, and early patient mortality (23, 24). Moreover, following the stabilization of RTRAF gene expression, progression and metastasis to lymph nodes were observed in both esophageal and pancreatic cancers (25, 26). In both low-grade and high-grade brain neoplasms, the RTRAF gene expression does not exhibit a noteworthy disparity, proposing that the RTRAF gene overexpression is of paramount importance in the genesis of tumors during the initial phases of tumorigenesis (14). As a concluding remark, the JAK2/STAT3 signaling pathway has been posited as the primary controller of the RTRAF gene (24).
There have been numerous suggested functions for the RTRAF gene, and alterations in its expression have been observed in tumors of various organs, including the bladder, breast, pharynx, cervix, pancreas, and esophagus. The authors noted that no comparable research has been conducted on thyroid cancer. This study presents the first evidence showing that the RTRAF gene expression is significantly reduced in benign thyroid tumors (goiters) compared to adjacent healthy tissues. Furthermore, the RTRAF gene expression was found to be indistinguishable between the malignant thyroid tumor (PTC tissue) and the adjacent healthy tissue. Considering the role that the RTRAF gene overexpression plays in cell cycle progression, it is conceivable that benign goiter tumors inhibit their growth by decreasing the RTRAF gene expression to prevent malignancy. Silencing of the RTRAF gene in bladder cells has been observed to provide further support, leading to a sudden reduction in cell proliferation (22). In addition, there was an increase in cells entering the G1 phase and a decrease in cells entering the S phase. The highlighted aspect pertains to the role of the RTRAF gene in promoting the growth of malignant cells. A decrease in the RTRAF gene expression in thyroid cancer may inhibit malignant growth. Benign tumors show a reduced RTRAF gene expression, which may allow for inhibiting cell cycle proteins and stopping proliferation.