1. Background
Breast cancer, a prevalent cancer, is responsible for the majority of women’s cancer deaths. Type I growth factor receptors, including the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor 2 (HER2), have been linked to reduced survival, tumor invasion, and metastasis in malignant breast cancer (1).
Mesenchymal stromal cells (MSCs) with immunomodulatory and neurotrophic characteristics make them a desirable choice for cell therapy (2). Suggestion advocates that the capability of MSCs to create substances that promote angiogenesis, incite host cells to repair injured tissues, and prevent apoptosis might be crucial for the protection of damaged tissue that results from these processes (3). Due to their distinct immunoregulatory and proliferative abilities, probably as a result of their early origin, Wharton’s jelly-derived mesenchymal stromal cells (WJ-MSCs) from the umbilical cord make particularly intriguing candidates for use in cell treatments (4). Additionally, because they can be collected using noninvasive techniques from a tissue that is typically discarded, WJ-MSCs have a simple and painless collection process (5).
Mesenchymal stromal cells-conditioned media (MSCs-CM) has a high concentration of cytokines and compounds, such as interleukins, growth factors, glycosaminoglycan, and cell adhesion molecules (6). The MSCs’ secretion of extracellular vehicles (EVs) containing various types of biomolecules has been recognized as a key sort of paracrine signaling (7). The secretome of MSCs (MSC-S) controls stem cell-to-cell communication, and a cell-free secretome can imitate stem cells’ immunomodulatory and tissue-regenerative capacities (8). Over the last decade, there has been a significant effort to analyze the impact of MSCs (including hWJ-MSCs) and their secretome on various illnesses, such as breast carcinoma, osteosarcoma, pancreatic, and lung tumor growth (9).
Damage caused by oxidative stress is involved in many types of diseases, including neurological diseases (Alzheimer’s and Parkinson’s), diabetes, atherosclerosis, arthritis, inflammation, and, most importantly, all types of cancer, such as breast cancer (10). Oxidative stress is actually an imbalance between the ratio of oxidants and antioxidants, during which a disturbance occurs in the body’s redox state and oxidation-reduction reactions (11, 12). This disorder is the result of an increase in free radicals, an imbalance between the production and elimination of active species in the body, and a decrease in the power of the antioxidant defense system. Many cellular processes, including cell metabolism, message transmission pathways, gene expression regulation pathways, cell proliferation, and programmed cell death, are affected by oxidative stress (13). The increase of free radicals causes changes in the structure and function of the body’s main biological molecules, including proteins, lipids, and nucleic acids, and ultimately leads to tissue damage. The products of this damage are used as biomarkers of oxidative stress in the evaluation and diagnosis of various cancers, such as breast cancer, as tumor markers (14).
Oxidative stress affects the antioxidant system, epithelial to mesenchymal transition (EMT) markers, and breast cancer cell metastasis (15). Mesenchymal stromal cells have anti-inflammatory and cytoprotective properties that might account for their success in the treatment of a variety of disorders (16). For this purpose, MSCs must be able to control the redox environment and lessen oxidative stress (17).
2. Objectives
This study investigated the effects of antioxidant, anti-proliferative, and anti-metastasis properties of human Wharton’s jelly human mesenchymal stem cell secretomes (hWJMSCs-Se) on the HER2-positive human mammary epithelial cell line SK-BR3.
3. Methods
3.1. Collection of Umbilical Cord
Umbilical cord samples were isolated from five newborn babies and women with healthy pregnancies after a cesarean section to identify Wharton’s jelly in the Department of Obstetrics and Gynecology of Abadan University of Medical Sciences, Ahvaz, Iran. For Wharton’s jelly sampling, investigational measures were accepted by the Ethics Committee of Abadan University of Medical Sciences guidelines (approval number: IR.ABADANUMS.REC.1399.132), and the owners have granted their written approval for tissue retrieval for scientific research. At four degrees Celsius, umbilical cord samples were collected in phosphate-buffered saline (PBS) containing 100 IU of penicillin per mL, 100 mg of streptomycin per mL, and 0.25 mg of amphotericin B per ml (Gibco, Grand Island, NY, USA). They were immediately transferred to a laboratory up to 3 hours after delivery.
3.2. Isolation of Wharton’s Jelly from Umbilical Cord
The human umbilical cord was thoroughly washed in PBS to remove potentially contaminated blood cells; then, two arteries and one vein were removed from each section’s blood vessels, and WJ was then carefully separated from the amniotic membrane. The remaining portion of the cord, known as the umbilical cord matrix, was cut into 1-mm segments and grown in a medium that contains 5 mL of full high-glucose Dulbecco Modified Eagle Medium (DMEM, Gibco, Grand Island, NY, USA), with 10% fetal bovine serum, 1% L-glutamine, and 1% penicillin/streptomycin solution. The cells were grown and maintained at 37°C for 9 - 10 days in an incubator with 5% CO2 for optimal growth and maintenance. Each explant’s edge saw the onset of cell growth. The cells were subcultured once they had achieved confluence up to 80%. The cells were identified at passage 3 through the use of specific surface markers.
3.3. Flow-Cytometric Analysis
After passage, in brief, 106 cells/ml of grown cells were submerged in cold PBS. The complex was examined by flow cytometry after being incubated with primary antibodies (CD34, CD44, CD90, and CD105) and secondary conjugated antibody for 30 minutes at 4°C. The FlowJo software (version 6.2) was used to analyze the data.
3.4. Secretome Preparation
Mesenchymal stem cells secrete a compound known as the secretome. After three days, the culture media for mesenchymal stem cells that had achieved passage 3 were collected. Following a PBS wash, the cells were given 20 hours of incubation in a serum-free medium. The dead cells were separated from the conditioned medium by centrifuging it at 2000 rpm for 5 minutes. The conditioned media was centrifuged once more with 10 mL at 2000 rpm for 2 hours at 4°C using Amicon Ultra-15 centrifugal filter devices with a 3000 molecular weight cutoff membrane.
3.5. SK-BR3 Cell Line Culture
The human breast cancer cell lines SK-BR3 were bought from the Pasteur Institute’s cell bank in Tehran, Iran. At 37°C and 5% CO2, tumor cells were raised in complete media composed of high-glucose DMEM, 100 U/ml penicillin, and 100 g/mL streptomycin. In order to treat the control and experimental groups of cells for 48 hours with 10, 25, and 50 g/mL of WJMSCs-Se (diluted in serum-free medium), the media was removed when the cells had reached 80% confluence. The effective concentration and incubation period of WJMSCs-Se exposure were selected based on the investigation of the half-maximal inhibitory concentration (IC50) by MTT assay.
3.6. MTT Assay
The MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H- tetrazolium bromide) assay was used to determine the cytotoxicity of hWJMSCs-Se. Triplicates of SK-BR3 cells were seeded in 94-well plates at a starting density of 4 × 103 cells per well. After 24 hours, the cells were exposed to hWJMSCs-Se or control media before being cultured for 1, 2, or 3 days. At each time point, a 0.5 mg/mL MTT solution was applied to each well, and each well was then given time to incubate at 37°C with 5% CO2 in a humid atmosphere. After four hours of incubation, the MTT reagent was removed, and 100 µL of dimethyl sulfoxide (DMSO) was added in its place. After 15 minutes of brief plate shaking at 600 rpm, the absorbance plate was measured at 570 nm wavelength on the plates using a Biotek EIX800 microplate reader (Winooski, USA). In GraphPad Prism (GraphPad Software, version 8, San Diego, CA), IC50 values were calculated from pharmacological dose-response curves. The output was entered as mean (SD or standard deviation). The cells were separated into three groups and exposed to different concentrations of hWJMSCs-Se for 48 hours: Blank (untreated), hWJMSCs-Se (10 µg/mL), hWJMSCs-Se (25 µg/mL), and hWJMSCs-Se (50 µg/mL).
3.7. Colony-Forming Unit (CFU) Assay
The SK-BR3 cells were plated in 6-well plates at a density of 500 cells per well at passage 2 for the colony-forming unit test, and they were given complete media augmented with 10, 25, and 50 µg/mL hWJMSCs-Se. For 14 days, the cells were cultured in full media. The cells were cleaned with PBS, fixed with 4% paraformaldehyde solution (Sigma Aldrich, St. Louis, MO, USA), and stained with 0.1% laboratory-grade crystal violet solution to make cell colonies visible. To count the number of colonies, the process was continued for 10 minutes, cleaned, and air-dried. The ratio of colonies to cells that were planted was used to define colony formation efficiency.
3.8. Apoptosis Assays by Annexin V/PI
The study examined the effect of hWJMSCs-Se on SK-BR3 cells using an apoptotic assay using annexin V/propidium iodide (PI) staining. The SK-BR3 cells were grown in DMEM containing hWJMSCs-Se for 48 hours. Annexin V-FITC/PI staining (Thermo Fisher Kit) was used to distinguish between healthy, necrotic, and apoptotic cells. Flow cytometry (Becton, CA, USA) was used to analyze the samples, classifying cells as alive, dead, early, or late. WinMDI 2.9 software was used for data analysis.
3.9. Antioxidative Enzyme Detection
The protein concentration of the cell homogenate was measured with an automated biochemical analyzer. The activities of superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) in cells were measured using SOD Assay Kit, CAT Assay Kit, and GSH Assay Kit (Nactaz™-Enzyme Activity assay kit, NavandSalamat, Iran), respectively. By normalizing the protein concentration in homogenates, the activities of SOD, CAT, and GSH were measured at the relative level.
3.10. Catalase Detection
Catalase activity can be determined based on the ability of this enzyme to break down H2O2 in cells. Briefly, 106 cell pellets were harvested, and 1000 μL of lysis buffer was added to the microtube for homogenization and centrifuged at 8000 g for 10 minutes at 4°C. The supernatant was used to measure the amount of CAT activity according to the relevant kit instructions. The absorbance of the sample and color development is achieved at a wavelength of 550 nm and can be read by a spectrophotometric instrument.
3.11. Superoxide Dismutase Detection
The study assessed total SOD activity by analyzing superoxide radicals produced by hypoxanthine and xanthine oxidase reacting with nitroblue tetrazolium to form a formazan dye. In essence, 106 cell pellets were harvested, 500 μL of lysis buffer was added to the microtube to homogenize, and 5 minutes were utilized for centrifuging at 11000 g at 4°C. The level of SOD activity was measured in the supernatant in accordance with the instructions of the commercial kit. Reduced color creation at 405 nm was used to test the SOD activity, which is considered to be inhibitory and might be measured with spectrophotometric equipment.
3.12. Glutathione Contents
Glutathione derivatives with the identifier (dithionitrobenzoate [DTNB]) and the generation of a yellow color, as said by the manufacturer’s instructions, indicate the glutathione content. Chiefly, 1000 μL of lysis buffer and 107 cell pellets were trypsinized and centrifuged at 9000 g for 15 minutes at 4°C to homogenize. Based on the commercial kit instructions, the supernatant was used to test glutathione levels. A spectrophotometric device can be used to read the absorbance rate at a wavelength of 412 nm.
3.13. RNA Extraction and Real‑time PCR
The extraction of ribonucleic acid (RNA) from cells was completed using the Favorgen Total RNA Isolation Kit (Favorgen, Taiwan), and RT (reverse transcription) was performed by the Complementary DNA (cDNA) Synthesis Kit (Yekta Tajhiz Azma, Iran). A reverse transcription reaction using a modified oligonucleotide (dT) was conducted to evaluate messenger ribonucleic acid (mRNA). The resulting cDNA was subjected to SYBR Green High ROX detection by quantitative polymerase chain reaction (qPCR) (Ampliqon, Denmark) using an Applied Biosystems Step OnePlus™ real-time PCR (initial denaturation: 15 minutes at 95°C; denaturation at 95°C for 15 seconds, 40 cycles, 60°C, annealing 1 minute). The relative frequency of genes with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was measured using the comparative Ct (2-ΔΔCt) method. Primers are listed in Table 1.
| Name | Forward | Reverse | Product Size |
|---|---|---|---|
| E-cadherin | GCTGGACCGAGAGAGTTTCC | CAAAATCCAAGCCCGTGGTG | 155 |
| β-catenin | GAGGCTTCTGGTGAAATCGC | TGCAGTTGCTAAACTTCACATT | 121 |
| N-cadherin | ACGGAGGAAGGTCTGAGGA | CCAACTCCATCAAATCAGCTTG | 179 |
| Vimentin | GGCGAGGAGAGCAGGATTTC | TGGGTATCAACCAGAGGGAGT | 95 |
| GAPDH | GGTCGGAGTCAACGGATTTGG | TGATGACAAGCTTCCCGTTCT | 194 |
Abbreviation: GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
3.14. Statistical Analysis
The obtained results were expressed as the mean ± standard deviation (SD). Two-way analysis of variance (ANOVA) and the Tukey test were used to analyze the means, and SPSS software (version 22) was used to determine the differences in parameters in all of the studied groups. Statistical significance was assessed by calculating P-values ≤ 0.05.
4. Results
4.1. Effective Inhibition of Cell Proliferation and Alteration of the Cellular Morphology of SK-BR3 Cells by hWJMSCs-Se
As seen in Figure 1, the results showed that hWJMSCs-Se could decrease total cell viability at the concentration manner in comparison to the control group when exposed to all three hWJMSCs-Se concentrates (10, 25, and 50 µg/mL) for 48 hours with the 50 µg/mL concentrate exerting the greatest reduction in cell viability. After 48 hours of incubation with several doses of the hWJMSCs-Se, it was observed that the survival rate had significantly diminished, from 100% in control to 68% and 56% in cells treated with 25 and 50 µg/mL, respectively. For the remainder of the investigation, 25 and 50 µg/mL were chosen at a time point of 48 hours. The untreated cells in Figure 1 were highly connected to each other; however, the cells in the hWJMSCs-Se treated groups (10, 25, or 50 µg/mL) showed dose-dependently fewer cellular connections. The aforementioned findings demonstrated that hWJMSCs-Se reduced cell growth and altered cellular shape.
Effects of human Wharton’s jelly mesenchymal stem cell secretomes (hWJMSCs-Se) on viability and morphology of the SK-BR3 cells; SK-BR3 cells treated with 25 or 50 μg/mL hWJMSCs-Se for 48 h. *** P < 0.001; * Symbol indicates the comparison of hWJMSCs-Se groups and non-treated controls. Results are presented as means ± standard deviation (SD) and are representative of three independent experiments.
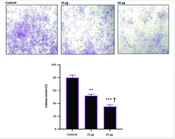
4.2. Repression of Colony Forming Tendency in SK-BR3 Cells by hWJMSCs-Se
The study demonstrated that varying doses of hWJMSC-Se can restrict or decrease the colony-forming tendency of SK-BR3 breast cancer cells, and this propensity is stronger as the dosage is increased. As a result, treatment with 50 μg/mL hWJMSCs-Se compared to cells treated with 25 μg/mL and control reduces the tendency of breast cancer cells to form colonies (Figure 2).
Colony formation and number of SK-BR3 cells in different groups; SK-BR3 cells treated with 25 or 50 μg/mL human Wharton’s jelly mesenchymal stem cell secretomes (hWJMSCs-Se) for 48 h. ** P < 0.01; *** P < 0.001; * Symbol indicates the comparison of hWJMSCs-Se groups and non-treated controls; † Symbol indicates the comparison between 25 and 50 μg/mL hWJMSCs-Se groups. Results are presented as means ± standard deviation (SD) and are representative of three independent experiments.
4.3. Measurement of Apoptosis of SK-BR3 Cells as a Result of Treatment with hWJSMCs-Se
Apoptosis induction in cancer cells by hWJSMCs-Se was investigated using flow cytometric analysis with annexin V/PI staining. There was a dose-dependent increase in the proportion of apoptotic cells after treatment with hWJSMCs-Se. In untreated SK-BR3 cells, 4% of the cells were in the early stages, and 10% were in the late stage. A 25 µg/mL dose of hWJSMCs-Se significantly increased both early (2-fold, P < 0.05) and late (1.3-fold, P < 0.01) apoptosis in cells when evaluated with the control group. The early (2-fold, P < 0.05) and late (1.7-fold, P < 0.01) apoptosis of SK-BR3 cells were both markedly increased by 50 µg/mL hWJMSCs-Se evaluated with the control group (Figure 3).
Human Wharton’s jelly mesenchymal stem cell secretomes (hWJMSCs-Se)-induced apoptosis of SK-BR3 cells treated with 25 or 50 μg/mL hWJMSCs-Se for 48 h; Representative dot plots of annexin V-FITC (X-Axis) versus PI (Y-Axis) fluorescence for control and experimental groups. * P < 0.05; ** P < 0.01; † P < 0.05; * Symbol indicates the comparison of hWJMSCs-Se groups versus non-treated controls. † Symbol indicates the comparison between 25 and 50 μg/mL hWJMSCs-Se groups. Results are presented as means ± standard deviation (SD) and are representative of three independent experiments.
4.4. Activity of Antioxidant Enzymes Increased by Treatment with hWJSMCs-Se
In order to study the antioxidant defense enzymes of SK-BR3 cells in culture in the presence of hWJSMCs-Se for 48 hours, the activity of CAT and SOD and glutathione content was tested. Treatment of cultured SK-BR3 with either 25 or 50 µg/mL of hWJSMCs-Se evoked a significant increase in the activity of all three antioxidant enzymes (Figure 4). Superoxide dismutase showed an increase of 1.5-fold (P < 0.05) with 25 µg/mL hWJSMCs-Se and 2.2-fold (P < 0.01) with 50 µg/ml hWJSMCs-Se. In the case of CAT, the increase of this enzyme activity was higher in the presence of higher hWJSMCs-Se concentrations, showing a 2.7-fold (P < 0.01) enhancement with 50 µg/mL. However, the dose-dependent increase was less marked for glutathione content, with a 1.1-fold and 1.7-fold (P < 0.05) increased activity in 25 and 50 µg/mL hWJSMCs-Se, respectively.
Superoxide dismutase (SOD), catalase (CAT), and glutathione analysis of SK-BR3 cell line exposed to 25 and 50 µg/mL of human Wharton’s jelly mesenchymal stem cell secretomes (hWJMSCs-Se) concentrate for 48 h. The activity of SOD and CAT was significantly increased in fold changes of 25 µg/mL (P < 0.05) and 50 µg/mL (P < 0.01) compared to controls. Glutathione content was significantly increased in fold changes of 50 µg/mL (P < 0.01) compared to controls. Results are presented as means ± standard deviation (SD) and are representative of three independent experiments
4.5. Real-time PCR
We looked into how hWJMSCs-Se affected the levels of E-cadherin, β-catenin, and different EMT markers, such as N-cadherin and Vimentin. The SK-BR3 cells were treated with 25 and 50 µg/ml of hWJSMCs-Se concentrations for 48 hours, after which gene expression levels were analyzed via real-time PCR. The hWJMSCs-Se concentration significantly dependently diminished the level of the E-cadherin gene while significantly increasing the Vimentin, N-cadherin gene level at a dose-response compared to the control (Figure 5). The obtained results demonstrated that EMT might be arrested by hWJMSCs-Se treatment.
E-Cadherin, β-Catenin, N-Cadherin, and Vimentin Gene Expression of SK-BR3 Cell Line Exposed to 25 and 50 µg/mL of Human Wharton’s Jelly Mesenchymal Stem Cell Secretomes (hWJMSCs-Se) Concentrate for 48 h.
E-cadherin was significantly decreased in fold changes of 50 µg/mL (P < 0.02) compared to controls. N-cadherin was significantly increased in fold changes of 50 µg/mL (P < 0.05) compared to controls. N-cadherin and vimentin were significantly increased in fold changes of 25 µg/mL (P < 0.05) and 50 µg/mL (P < 0.01) compared to controls. Results are presented as means ± standard deviation (SD) and are representative of three independent experiments.
5. Discussion
Despite improvements in diagnosis and treatment, breast cancer still ranks as the number one cause of cancer-related death in women globally (18). This study examined the potential of hWJMSCs-Se in reducing the effects of oxidative stress associated with breast cancer, specifically SK-BR3 cells.
It is well known that oxidative stress plays an important role in the onset and spread of cancer. Reactive oxygen species (ROS) can damage DNA, proteins, and lipids in cells, which can result in genetic abnormalities, aberrant cell signaling, and the promotion of tumors (19). This work highlights the importance of oxidative stress in breast cancer biology and its therapeutic potential.
Mesenchymal stromal cells secrete a variety of bioactive compounds, including growth factors, cytokines, and extracellular vesicles, which have recently gained interest (20). A number of factors, including ease of collection, low immunogenicity, and regenerative potential, make Wharton’s gel-derived MSCs promising (21). The MSC-related immunomodulatory and tissue gene-generating properties are the focus of the study’s attention on hWJMSCs-Se, a cell approach (22).
The findings demonstrated that hWJMSCs-Se therapy significantly lowers the viability and proliferation of SK-BR3 breast cancer cells. This finding implies that hWJMSCs-Se can prevent cancer cells from proliferating. The fact that this inhibition was time- and dose-dependent is significant since it suggests the possibility of individualized treatment approaches.
The induction of apoptosis in SK-BR3 cells after hWJMSCs-Se treatment is one of the main findings of the study. Apoptosis, a method of programmed cell death, is often blocked in cancer cells. Human Wharton’s jelly mesenchymal stem cell secretomes have the ability to induce selective death of cancer cells and spare healthy cells, as evidenced by its ability to accelerate apoptosis of breast cancer cells (23).
The present study highlights the potential of hWJMSCs-Se in reducing proliferation and inducing apoptosis in SK-BR3 cells. Widowati et al. recently proposed that hWJMSCs effectively inhibited proliferation in T47D and MCF7 cell lines, with an IC50 value of 42.34% on MCF7 and 42.36% on T47D, and hWJMSCs significantly induced apoptotic genes P53, BAX, and caspase 9 expression in MCF7 and T47D cells, with no significant decrease in antiapoptotic gene BCL-2 (24).
In contrast to the current study’s results, H. Sadeghi-aliabadi’s study reported limited effectiveness of WJMSC secretome therapies in inducing apoptosis and proliferation of A549 lung cancer cells.
Their response to doxorubicin showed no significant effects, suggesting the need for further investigation into the mechanisms underlying this phenomenon (25).
Superoxide dismutase and catalase (CAT), two oxidation enzymes, can lower excessive ROS, a sign of oxidative stress (26). This study showed that hWJMSCs-Se treatment significantly boosted the activity of these antioxidant enzymes in SK-BR3 cells. As to the current investigation, HWJMSC-SE might increase cell survival, reduce oxidative stress, and assist cancer cells in regaining their redox balance.
Disrupted redox balance is linked to angiogenesis, metastasis, and the growth of cancer in human cells. Mesenchymal stromal cells offer a superior alternative for treating ROS-induced damage linked to chronic diseases, such as cancer, dementia, and kidney failure, due to their therapeutic and regenerative capabilities (27). Studies have suggested that MSCs might be used to treat specific disorders brought on by ROS (27), which is consistent with the present study’s findings about the antioxidative properties of hWJMSCs-Se. Since MSCs can renew, regulate the immune system, and migrate, they represent a potential next-generation treatment with no adverse effects.
An essential step in the cancer mutase process is EMT (28). The research shows that hWJMSCs-Se therapy results in alterations in EMT marker genes. Vimentin and N-cadherin expression are upregulated, although E-cadherin and -catenin levels are downregulated specifically. These are mesenchymal indicators. This finding shows that using hWJMSCs-Se to treat breast cancer cells might block the EMT process, potentially lowering the likelihood of breast cancer cells invading and spreading.
The process of ROS-induced EMT, which is essential for metastasis, might be connected to the development of chemoresistance (29). It is worth noting that Ozge Cevik conducted WJMCS exosomes to suppress EMT signaling in cervical cancer cells as an effective paclitaxel drug delivery system (30).
The findings of this study offer hope for the creation of novel therapies for HER2-positive breast cancer. Human Wharton’s jelly mesenchymal stem cell secretomes are interesting candidates for future targeted cancer therapies because they lower oxidative stress, prevent cell proliferation, induce apoptosis, and modify EMT pathways. Its non-invasive collecting method and immunomodulatory capabilities further boost its attractiveness as a therapeutic agent.
5.1. Conclusions
In conclusion, this study emphasizes hWJMSCs-Se’s ability to lessen the impacts of oxidative stress-induced breast cancer. The observed effects on cell survival, apoptotic induction, augmentation of antioxidant enzymes, and modification of EMT indicators demonstrate the broad therapeutic potential of hWJMSCs-Se. All the therapeutic implications of this novel strategy for treating breast cancer require further investigation and clinical trials.