1. Background
Glioma, the predominant type of tumor in the central nervous system (CNS), originates from glial cells (1). Various therapeutic methods have been employed to treat glioma, including temozolomide chemotherapy, radiation therapy, and surgery (2). Temozolomide is an alkylating agent that induces mutations in DNA strands, resulting in cell cycle arrest and apoptosis. However, chemotherapy resistance can occasionally lead to treatment failure and cancer recurrence (2-4).
Therefore, to enhance treatment strategies for glioma, it is essential to comprehend its fundamental biology and the molecular factors contributing to drug resistance. Recent research has been centered on the tumor microenvironment (TME), recognized as a critical element in drug resistance. Tumor microenvironment components can trigger intracellular and intercellular signals that regulate tumor development, progression, metastasis, and treatment response. Consequently, grasping the biological interactions between cancer cells and their surroundings is of utmost importance. These experiments may offer insights into combinatorial therapy and the long-term survival of glioma patients (5-7).
According to the findings, tumor cells exhibit a distinct glycosylation pattern (8). Sialylation is a form of terminal glycosylation that regulates numerous cellular and biological processes. It involves the attachment of sialic acid to glycoproteins and glycolipids (9). Sialic acid has an impact on the structure and stability of glycoconjugates, as well as interactions between cells, including immune recognition (10). Abnormal sialylation has been implicated in the development, progression, invasion, and metastasis of various cancers, including breast cancer, gastric cancer, and glioma (9, 11).
Studies have revealed that disruptions in glycosylation and sialylation can profoundly affect the function of drug transporters, resulting in changes in drug absorption or efflux. This can potentially contribute to chemotherapy resistance. Multiple studies suggest that impaired glycosylation of ABC transporters, such as ABCC1 and multidrug resistance-associated protein (MRP)4, can lead to decreased cell surface expression and altered subcellular localization, thereby contributing to drug resistance in cancer cells. Consequently, further investigations into the impact of sialylation on drug transporters may uncover new mechanisms of resistance and offer novel targets for therapeutic interventions (12).
Increased expression and enhanced effectiveness of drug efflux pumps, particularly those belonging to the ATP-binding cassette (ABC) superfamily, represent another frequently observed mechanism of drug resistance (13, 14). The ABC transporters constitute a protein superfamily with a long evolutionary history found in all living organisms (15). Within the human genome, there are a total of 48 ABC genes, classified into seven subfamilies, denoted as ABCA to ABCG. Notably, ABCB1, ABCC1, and ABCG2 have been identified as major contributors to the development of multidrug resistance (MDR) in response to cancer chemotherapy drugs (16).
P-glycoprotein (P-gp), also referred to as ABCB1 or MDR1 is an ABC transporter that plays a pivotal role in cellular detoxification by actively expelling xenobiotic compounds. P-glycoprotein is typically present in barrier tissues like the blood-brain barrier, liver, and placenta, where it safeguards vital organs by preventing the buildup of potentially harmful substances. This transporter significantly influences the pharmacokinetics of numerous drugs and is linked to chemotherapy resistance in cancer cells (17, 18). According to reports, patients with tumors expressing ABCB1 are three times more likely to experience treatment failure compared to those with tumors lacking ABCB1 expression (19).
ABCC1, also known as MRP1, represents a ubiquitous ABC transporter present in all human tissues. It plays a pivotal role in facilitating cellular detoxification and the elimination of potentially harmful compounds from within the cell (20). This transport mechanism has been linked to chemoresistance and adverse clinical outcomes in patients with neuroblastoma. Additionally, it has been associated with increased tumor severity and poorer patient outcomes in both primary and recurrent gliomas (21). ABCC1 is often found to be overexpressed in various types of tumors, including esophageal cancer, classical Hodgkin lymphoma, lung cancer, and colorectal cancer. This heightened expression has been correlated with resistance to a wide range of anticancer medications (20, 22). These findings underscore the significance of ABCC1 in mediating drug resistance and underscore the need for strategies to overcome this resistance and enhance treatment outcomes (23).
The overexpression of these ABC transporters empowers cancer cells to actively expel chemotherapy drugs from the intracellular environment. This action reduces the cytotoxic effects of the drugs, resulting in therapeutic failure and the survival of drug-resistant cancer cells. Therefore, comprehending the role of these transporters in drug distribution and resistance is crucial for conquering drug resistance. It is essential to note that drug resistance is a complex phenomenon, and its development may be influenced by various mechanisms. Other factors, such as modifications in drug targets, metabolic changes, and epigenetic alterations, may also contribute to chemoresistance (13, 14).
2. Objectives
In the current study, we evaluated the effects of temozolomide, sialic acid, and their combination (TMZ + sialic acid) on the viability and morphological characteristics of 1321N1 cells. Additionally, we assessed the expression of ABCB1 and ABCC1 using real-time PCR.
3. Methods
3.1. Cell Line and Cell Culture
We obtained the 1321N1 cell line from the National Cell Bank of Iran at the Pasteur Institute in Tehran. The cells were cultured in a DMEM low-glucose medium from Gibco, USA, supplemented with 10% fetal bovine serum (FBS) also from Gibco, USA. Additionally, 1% of each penicillin (5000 U/mL) and streptomycin (5000 mg/mL) were added to the medium. The cells were maintained in a controlled incubation environment at 37°C, 5% CO2, and 95% humidity.
3.2. MTT Cell Viability Assay (IC50 and EC50 Assessment)
We obtained temozolomide (TEMOZOLOMIDE ACTE 20 mg Capsule) from Acteropharma company (Iran) and sialic acid (N-acetylneuraminic acid: NANA) from Sigma-Aldrich company (Germany). We assessed cell viability using the MTT assay. Specifically, we seeded 5 × 103 1321N1 cells per well in a 96-well plate and incubated them under standard conditions for 24 hours. To determine the IC50 for temozolomide, we treated cells with concentrations ranging from 50 to 800 µM and 10 to 1000 µM for 48 and 72 hours, respectively (2). To establish the EC50 for sialic acid, cells were exposed to concentrations ranging from 1 to 600 µM and 1 to 300 µM. For the IC50 assessment, cells were treated with sialic acid concentrations ranging from 600 to 2000 µM and 300 to 2000 µM for 48 and 72 hours, respectively. Lastly, for assessing changes in IC50 for temozolomide in combination with sialic acid, cells were treated with twice the half-maximal effective concentration (2*EC50) of sialic acid along with increasing concentrations of temozolomide for each time point. The IC50 and EC50 values were subsequently calculated using an MTT solution, DMSO, and an ELISA reader.
3.3. RNA Extraction and Real-time Quantitative PCR (RT qPCR)
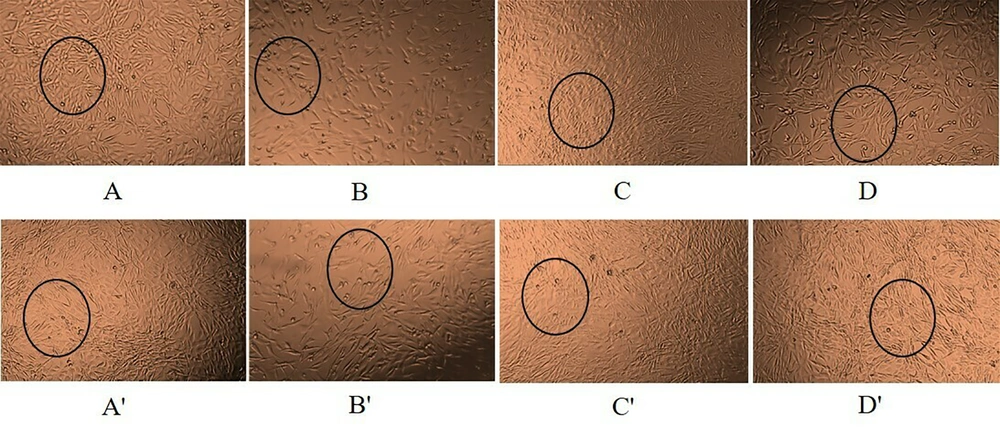
The cells were divided into four groups: Control, temozolomide, sialic acid, and temozolomide + sialic acid. Six-well plates were seeded with 13 × 104 cells per well. After 24 hours, cancer cells were treated with temozolomide, sialic acid, and temozolomide + sialic acid for 48 and 72 hours at concentrations equivalent to the IC50 of temozolomide and twice the EC50 of sialic acid. The cells were then observed and photographed using an inverted microscope (Figure 1). Subsequently, total RNA extraction was carried out following the manufacturer's protocol using QIAzol (Qiagen, Germany) reagent. The quality and quantity of RNA were assessed through electrophoresis on agarose gel and a NanoDrop instrument. Next, reverse transcription was conducted using 2000 ng of RNA and the Yektatajhiz cDNA synthesis kit (Iran) according to the manufacturer's instructions. Quantitative real-time PCR was used to evaluate the expression of ABCB1 and ABCC1 in 1321N1 cells, with the amplification primers listed in Table 1.
A and A', cells with no treatment; B and B', cells treated with IC50 of temozolomide; C and C', cells treated with 2*EC50 of sialic acid; D and D', cells treated with temozolomide in combination with sialic acid at the same concentrations after 48 and 72 h of treatment, respectively. All images were captured at x4 magnification.
| Primer | Sequence (5’ → 3’) | TM | Product Size (bp) |
|---|---|---|---|
| ABCB1 | 220 | ||
| F | TGGCCTTCTGGTATGGGAC | 58.69 | |
| R | GGTTTGTGCCCACTCTTCG | 59.05 | |
| ABCC1 | 128 | ||
| F | GGAGGACACGTCGGAACAA | 59.64 | |
| R | AACTCTCTTTCGGCTGGGC | 60.00 | |
| ACTB | 103 | ||
| F | GAGCATCCCCCAAAGTTCACA | 60.55 | |
| R | GGGACTTCCTGTAACAACGCA | 60.54 |
3.4. Statistical Analysis
All experiments were conducted in duplicate, and data collected from two independent experiments were presented as mean ± standard deviation (SD). Statistical analyses were performed using GraphPad PRISM 9 and evaluated using a one-way ANOVA test. A statistically significant result was defined as a P-value less than 0.05.
4. Results
4.1. Cell Viability
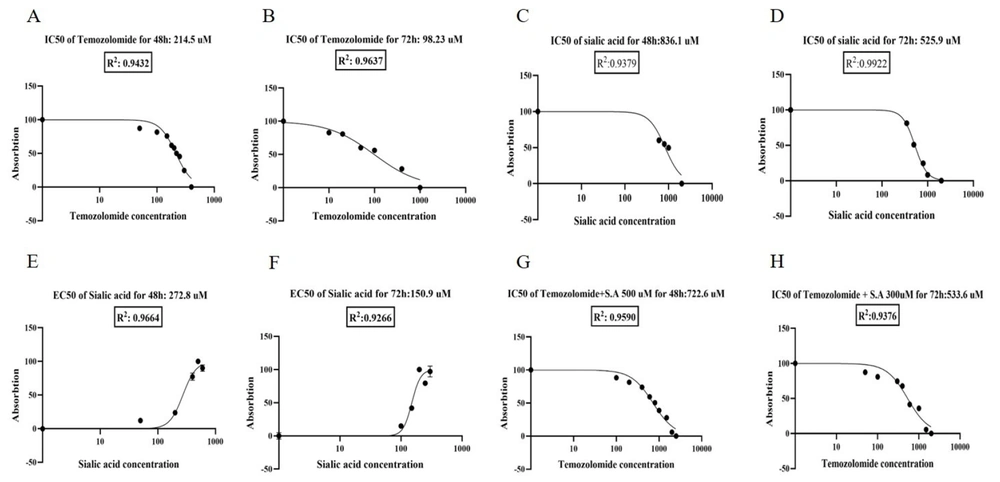
In our study, the IC50 of temozolomide was determined to be approximately 215 μM for 48 hours and 100 μM for 72 hours (Figure 2A and B). We evaluated the metastatic potential of sialic acid by examining its EC50 in a concentration and time-dependent manner. Our findings demonstrate that the administration of sialic acid significantly enhances the survival rate of the 1321N1 cell line, as indicated by the MTT assay. Consequently, IC50 values of sialic acid were observed to be approximately 835 μM and 525 μM. Additionally, the EC50 values of sialic acid were roughly 270 μM and 150 μM after 48 and 72 hours of treatment, respectively (Figure 2C - F). According to the MTT results, the anticancer effect of temozolomide was modulated in a time- and concentration-dependent manner when combined with sialic acid treatment. Therefore, the IC50 of temozolomide in combination with sialic acid treatment was defined as about 720 μM for 48 hours and 530 μM for 72 hours, respectively (Figure 2G and H). The findings of this research indicate that the IC50 values of temozolomide have shown a notable increase of 3-fold and 5-fold after 48 and 72 hours, respectively, upon exposure to 2*EC50 of sialic acid. This evidence suggests that the cells have acquired resistance to the drug due to the presence of sialic acid in their microenvironment.
4.2. Morphology Analysis by an Inverted Microscope
After treatment with temozolomide, significant changes in cellular morphology were observed, including reduced cell size and number, increased programmed cell death, and increased distances between cells compared to the control group (Figure 1A, A', B, and B'). Sialic acid treatment caused significant changes in cellular characteristics, including increased size and hypercellularity, reduced mortality, and elevated cell aggregation with less extracellular space (Figure 1A, A', C, and C'). The combination of temozolomide and sialic acid treatment also resulted in significant changes in cell characteristics compared to temozolomide alone, including increased cell size and number, decreased apoptosis, and increased cellular accumulations. Interestingly, the cells behaved similarly to the control cells (Figure 1A, A', D, and D'). According to the findings of this research, it is suggested that sialic acid has the ability to increase cellular drug resistance and diminish apoptosis. In summary, our team's extensive research has highlighted the crucial role of sialic acid in cellular processes such as growth, angiogenesis, adhesion, metastasis, and invasion. Therefore, understanding the role of sialic acid is crucial for unraveling biological mechanisms and developing targeted cancer therapies (24).
4.3. Effect of Temozolomide and Sialic Acid Treatment on the Relative Expression of ABCB1 and ABCC1 Genes in the 1321N1 Cell Line
4.3.1. ABCB1 Expression
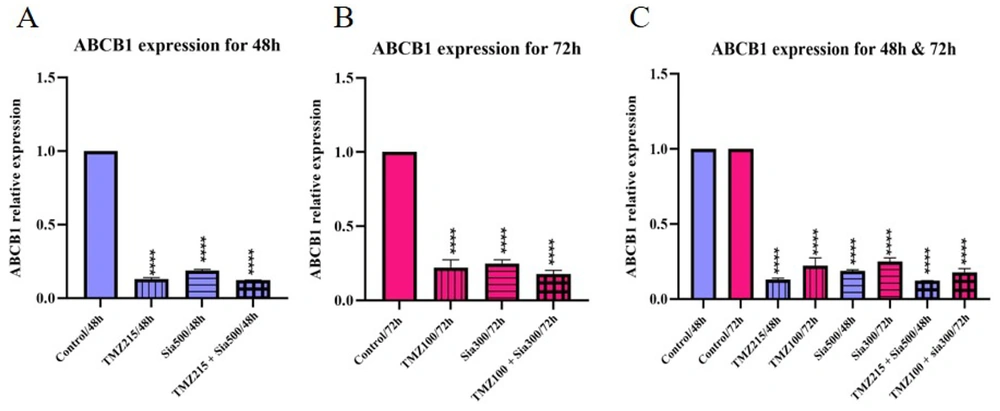
The investigation found that both sialic acid and temozolomide caused a significant decrease in the expression of the ABCB1 gene at 48 and 72 hours. Sialic acid alone led to a decrease compared to the control group but an increase compared to other treatments. Temozolomide alone also reduced gene expression, and when combined with sialic acid, there was an even greater decrease. The time and concentration of the treatments influenced the expression of the ABCB1 gene, with higher levels of expression observed at 72 hours (Figure 3).
The ABCB1 gene was analyzed in 1321N1 through qRT-PCR expression analysis. A, the cells were treated with TMZ, sialic acid, and TMZ + sialic acid, while untreated cells were used as the control. The IC50 of temozolomide (215 uM) and 2*EC50 of sialic acid (2*270 uM) on 1321N1 were used for 48 h; B, the cells were treated with TMZ, sialic acid, and TMZ + sialic acid, while untreated cells were used as the control. The IC50 of temozolomide (100 uM) and 2*EC50 of sialic acid (2*150 uM) on 1321N1 were used for 72 h; C, A and B (**** P-value < 0.0001).
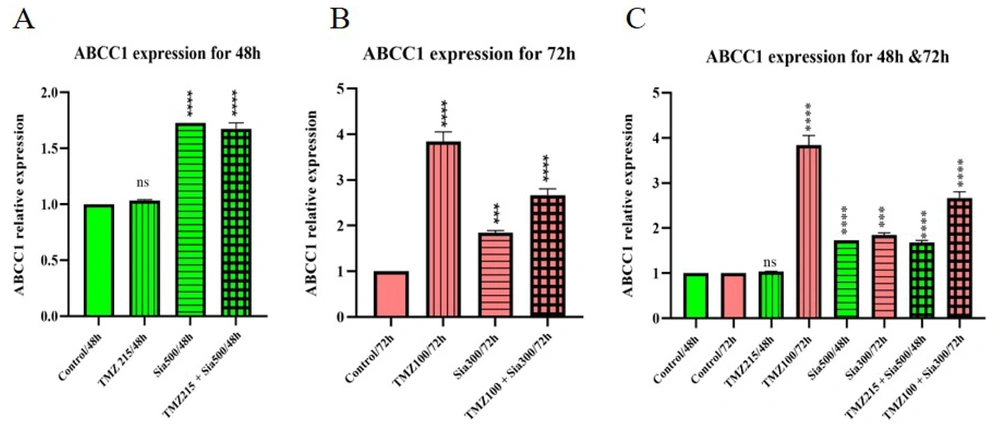
4.3.2. ABCC1 Expression
The analysis of ABCC1 gene expression in cells treated with sialic acid and temozolomide reveals a significant increase in gene expression compared to the control group. Specifically, temozolomide treatment leads to a 3.8-fold increase in ABCC1 gene expression after 72 hours. Sialic acid, whether used alone or in combination with temozolomide, also upregulates ABCC1 gene expression. This finding is significant because the ABCC1 gene is associated with drug resistance, and its expression is influenced by both time and concentration (Figure 4).
The ABCC1 gene was analyzed in 1321N1 through qRT-PCR analysis. A, the cells were treated with TMZ, sialic acid, and TMZ + sialic acid, while untreated cells were used as the control. The IC50 of temozolomide (215 uM) and 2*EC50 of sialic acid (2*270 uM) on 1321N1 were used for 48 h; B, the cells were treated with TMZ, sialic acid, and TMZ + sialic acid, while untreated cells were used as the control. The IC50 of temozolomide (100 uM) and 2*EC50 of sialic acid (2*150 uM) on 1321N1 were used for 72 h; C, A and B (*** P-value < 0.001 and **** P-value < 0.0001).
5. Discussion
Cancer represents a major global health challenge, and chemotherapy stands as the most frequently employed treatment method. Unfortunately, this therapy does not substantially enhance patient outcomes, as 70 - 80% of advanced cancer patients encounter tumor recurrence, resulting in more severe disease states and, often, fatal consequences. One of the primary reasons for disease recurrence is the survival of cells capable of withstanding treatment, which endure the initial therapeutic intervention. These resilient cells serve as the groundwork for the development of future relapses (25). The role of epigenetic mechanisms in the TME has been the subject of extensive research, and their involvement in tumor growth has been well-documented. Drug resistance in glioma cells is a complex phenomenon, with various pathways employed to evade apoptosis induced by temozolomide (12).
Experimental evidence suggests that the TME possesses the capability to initiate cell signaling, thereby regulating tumor angiogenesis, cancer progression, and resistance to therapy (26). Within the TME, glycan networks, a prominent component, envelop cells, promote responses to the extracellular environment, evade detection by the immune system, and facilitate metastasis (7, 8, 27). Glycans play a pivotal role in mediating cell-cell recognition, communication, aggregation, and development, as well as regulating interactions between carbohydrates and proteins within organisms. Sialic acids, a type of glycan, hold a crucial role in tumor growth and metastasis and exert notable effects on immunology, cell signaling, reproduction, and nervous system biology (28). In cancer cells, the expression of sialic acids is often disrupted, leading to alterations in cell adhesion properties and immune surveillance, thereby promoting cancer progression. Recent research has revealed increased sialic acid levels in various types of cancer, including breast, ovarian, and colorectal cancer (29-31). The hypothesis is that sialic acid may play a pivotal role in the development of drug resistance by serving as a significant component within the architecture of cellular and molecular signaling crosstalk (6). Furthermore, disruptions in glycosylation and sialylation can impact ABC transporter genes, potentially leading to chemotherapy resistance. Therefore, identifying key elements in tumor development is crucial for advancing diagnostic techniques and treatments. Consequently, sialic acids have become a focal point of investigation in cancer biology, with researchers exploring their potential as diagnostic markers or targets for therapeutic interventions.
The results of this study suggest that the incorporation of sialic acid into temozolomide significantly increases the IC50 value of temozolomide at 48 and 72 hours, in contrast to temozolomide treatment alone. Furthermore, cell morphology analysis revealed that cells treated with temozolomide in combination with sialic acid behaved similarly to untreated cells. Notably, these cells exhibited lower levels of apoptosis compared to cells treated with temozolomide alone.
On the other hand, molecular biological analysis showed that ABCB1 expression was decreased, and ABCC1 expression was increased in all treatment groups at 48 and 72 hours. Temozolomide treatment had the most significant effect on gene expression compared to sialic acid alone or in combination. In fact, there were no significant changes in gene expression after 72 hours of sialic acid administration alone compared to 48 hours. The ability of sialic acid to modulate gene expression, either alone or in combination with temozolomide, is indicated by its capacity to induce slight changes at 72 hours. This finding contrasts with the gene expression changes observed at 48 hours.
Furthermore, sialic acid may regulate drug resistance by impacting the expression, structure, and function of transporter genes through direct or indirect mechanisms. Therefore, measuring protein production and activity is important to understand the differences in gene expression of ABC transporters, as sialic acid may affect drug resistance through other molecular pathways. Hence, comprehending the mechanisms leading to the downregulation of ABCB1 and upregulation of ABCC1 is essential for developing effective strategies to reverse TMZ resistance in glioma cells. It is important to consider that the presence of sialic acid in the brain, especially in glioma, may interfere with the effects of anticancer drugs by modulating the cellular microenvironment. As a result, the use of sialic acid inhibitors in combination with anticancer drugs like temozolomide has emerged as a promising strategy for multidrug therapy.
5.1. Conclusions
Our research demonstrates that the presence of sialic acid increases cancer growth and interferes with the functionality and efficacy of anticancer drugs over an extended period, ultimately resulting in ineffective treatment. Therefore, it is essential to focus on sialic acid or its production factors for therapeutic purposes. Consequently, the use of combination therapy is recommended for effective cancer treatment. This study provides a theoretical and empirical basis for understanding the effects of sialic acid on glioma cells and may lead to the identification of therapeutic targets for glioma treatment.