1. Background
Severe disorders during pregnancy encompass a broad spectrum of conditions that can cause abnormalities and mortality in both the mother and her fetus. It is estimated that these severe abnormalities occur in 6-8% of all pregnancies (1, 2). The incidence of preeclampsia (PE) in nulliparous and multiparous women is approximately 3 - 7% and 1 - 3%, respectively (3). A significant portion of these disorders is associated with hypertension-related complications during pregnancy, with PE being a prevalent disorder and a specific syndrome of pregnancy. Various factors, including genetic traits, underlying medical conditions, history of pregnancy, chronic hypertension, coagulation disorders, renal diseases, diabetes, autoimmune diseases such as lupus, a family history of PE, absence of family planning, twin or multiple pregnancies, age below 18 or above 35, among others, may contribute to its development. However, the exact contribution of these factors to PE has not been completely clarified (4-7).
Vascular endothelial growth factor (VEGF) plays a crucial role in the proliferation, differentiation, migration, and invasion of endothelial cells (1). Maintaining endothelial cell function is vital for the development and regulation of angiogenesis. The VEGF receptor (VEGF-R) primarily mediates biological functions through VEGF-R2 (2). The human VEGF gene is located on chromosome 6p21.3, spans 28 Kb in full length, and comprises 14 Kb of coding sequence, including eight exons and seven introns (3).
The involvement of VEGF and its receptor system is crucial for fetal growth and angiogenesis, significantly impacting fetal development. Slight alterations in VEGF expression during fetal development can result in abnormalities or fetal demise (5). Several studies suggest that polymorphisms in the VEGF gene play a critical role in regulating protein expression and enhancing susceptibility to PE, though evidence on this matter remains limited (6-8). Given the genetic diversity of the Iranian population compared to others, VEGF mutations in Iranian pregnant women might differ from those in different communities. Investigating these genes in relation to PE could yield distinct outcomes within the Iranian genetic context. Additionally, examining serum VEGF levels in women with PE, alongside polymorphisms of SNPs (-634C/G and +936C/T), holds significant relevance.
2. Objectives
Consequently, this study aimed to assess serum VEGF levels in women with PE and explore the association between -634C/G and +936C/T polymorphisms of the VEGF gene and the development of this condition in Ahvaz, Iran.
3. Methods
3.1. Study Population and Design
In this case-control study, peripheral blood samples were collected from 135 women diagnosed with PE and 135 normal pregnant women serving as the control group. The participants, aged between 18 and 37 years, were randomly selected from those attending the women’s wards of two hospitals affiliated with Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Women with PE who had a history of high blood pressure (BP), pre-pregnancy bleeding, multiple gestations, abortion, diabetes mellitus, chorioamnionitis, renal diseases, cardiovascular diseases, cigarette use, obesity, and activated autoimmune diseases were excluded from the study. A gynecologist diagnosed PE through clinical examinations and laboratory tests. This study included only women who provided their informed consent.
3.2. Requirements for the Patient and Control Groups
Women diagnosed with PE, exhibiting a systolic blood pressure of ≥ 140 mmHg and diastolic BP of ≥ 90 mmHg, and showing proteinuria of 300 mg or more in a 24-hour urine collection or proteinuria of 30 mg/dL in two urinary samples developed after the 20th week of pregnancy (9), were selected as the case group. The control group consisted of 135 healthy pregnant women from Khuzestan Province who had at least one alive and healthy child and no history of high BP during pregnancy. The age range for the women was 18 to 37 years. In this study, control group participants had no history of chronic kidney disease, high BP, or bleeding before pregnancy. The average blood collection time for the control group was 36.01 ± 1.75 weeks of gestation. All PE cases were identified in the third trimester of gestation. Blood samples from PE patients were collected before the initiation of antihypertensive drug treatment.
3.3. Sampling
Patients received instructions from a nurse. In sterile conditions, 7 mL of blood was drawn from each patient, with 4 ml being transferred into EDTA vials (Hakim Tajhiz Gostar, Iran) for DNA extraction. The extracted DNA samples were then stored at -20°C until analysis. Additionally, 3 ml of the remaining blood was deposited into a sterile clotting tube for the determination of serum VEGF concentration using the ELISA method (eBiosource, Cat. Number: BMS277/2CE, Germany). After centrifugation for serum separation, the serum was preserved at -70°C for further analysis. Blood samples from PE patients were collected before starting antihypertensive medication treatment.
3.4. DNA Extraction and Genotyping
DNA Extraction and Genotyping: Genomic DNA samples were extracted from leukocytes in whole blood samples collected in EDTA anticoagulated micro-tubes, following the method outlined by Miller et al. (10). For this project, primers 5'-AAGGAAGAGGAGACTCTGCGCAGAGCA-3' (forward) and 5'-TAAATGTATGTATGTGGGGTGTGTCTACAGG-3' (reverse) were utilized in PCR-RFLP to analyze the SNP +936C/T polymorphism. Additionally, to study the SNP -634C/G polymorphism, primers 5'-TTGTTCCCCACTCARTGATCG-3' (forward) and 5'-CCGTCAGCGCGACTGGTCA-3' (reverse) were employed (11). The PCR protocol included an initial denaturation step of 5 minutes at 95°C, followed by 50 seconds at 53°C and 30 seconds at 72°C, then 32 cycles consisting of a second denaturation phase at 94°C for 40 seconds, an annealing phase at 62°C for 60 seconds, and an extension phase at 72°C for 60 seconds. A final synthesis phase lasted 10 minutes at 72°C. Following PCR completion, the products underwent electrophoresis (12). To identify the +936C/T polymorphism, 1 μL of NlaIII restriction enzyme (Fermentas, USA) was added to the PCR product and incubated for 37 hours at 18°C. If the target sequence for this enzyme was present in the DNA, the original 208bp DNA strand was cleaved into two fragments of 122 bp and 86 bp by the enzyme's activity, and these fragments were then distinguished by their separation on a gel after electrophoresis (Bio-Rad, USA). Similarly, to assess the -634C/G polymorphism, 1 μl of FaqI restriction enzyme (Fermentas, USA) was added to the PCR product and incubated for 37 hours at 18°C. If the DNA contained the enzyme's target sequence, the original 304 bp DNA strand was divided into two pieces of 193 bp and 111 bp (13).
3.5. Quantification of VEGF Concentrations in Serum
The measurement of VEGF protein in serum samples was conducted using a specific enzyme-linked immunosorbent assay (ELISA) technique (VEGF Kit; eBioSource, Cat. Number: BMS277/2CE, USA) following the double-antibody sandwich method as per the manufacturer’s instructions. The color intensity, reflecting the concentration of VEGF protein in the serum samples, was measured at 450 nm and 630 nm using an ELx800™ ELISA Reader (BioTek, Winooski, VT, USA). Serum VEGF levels were determined based on standard curves, with VEGF concentrations reported in pg/ml.
3.6. Statistical Analysis
The Mann-Whitney test was applied to compare the expression results of the -634C/G and +936C/T polymorphisms in the VEGF gene and their correlation with serum VEGF levels in pregnant women with PE. To assess the relationship between these two polymorphisms and serum VEGF levels, both the Pearson correlation coefficient and the Spearman test were utilized. A confidence level of 55% was adopted for all calculations, and a p-value of less than 0.05 was considered statistically significant.
4. Results
According to the results of descriptive statistics, there was a significant difference in demographic and laboratory variables between normal pregnant women and women with PE, categorized as control and case groups, respectively (Table 1). Maternal age, maternal height, and BMI, as well as the average weight of women with PE, were higher than those in the control group. However, concentrations of hemoglobin, birth weight, and gestational age at birth were lower in women with PE compared to normal pregnant women (P < 0.05).
| Variables | Case (n = 135) | Control (n = 135) | P-Value |
|---|---|---|---|
| Mother's age (y) | 18 - 37 (28.6 ± 4.29) | 18 - 36 (26.3 ± 4.83) | < 0.05 |
| Mother's height (cm) | 1.5 - 1.7 (1.64 ± 0.034) | 1.5 - 1.7 (1.62 ± 0.039) | < 0.05 |
| Mother's weight (kg) | 68 - 96 (80.8 ± 4.38) | 60 - 81 (71.2 ± 4.06) | < 0.05 |
| Mother's body mass index (kg/m)2 | 24.4 - 37.5 (30.07 ± 1.97) | 23.7 - 32.4 (27.25 ± 1.48) | < 0.05 |
| Pregnancy week | 25 - 39 (34.35 ± 2.24) | 34 - 38 (36.52 ± 1.03) | < 0.05 |
| Birth weight (g) | 2000 - 3750 (2586.6 ± 427.4) | 3200 - 3540 (2934.8 ± 372.5) | < 0.05 |
| Haemoglobin (g/dL) | 9.1 - 13.3 (10.01 ± 0.75) | 9.5 - 13.6 (11.71 ± 0.8) | < 0.05 |
| Systolic blood pressure (mmHg) | 141 - 157 (144 ± 5.4) | 111 - 134 (120 ± 9) | < 0.05 |
| Diastolic blood pressure (mmHg) | 92 - 106 (95 ± 7.2) | 69 - 84 (77 ± 7) | < 0.05 |
| Proteinuria, g/24 (h) | 1.08 - 1.42 (1.31 ± 0.6) | Absent | - |
Demographic Data of Case and Control Groups a
The analysis of the relationship between the sample type and the season of PE occurrence revealed no association between the sample type and the season in which PE occurred (r = 0.523; P = 0.914).
The study found no correlation between the incidence of PE and any alleles related to SNP -634C/G and SNP +936C/T (P > 0.05). Considering the odds ratio, it was concluded that the likelihood of carrying G and C alleles in both the control and PE groups is identical, implying that possession of either the C or G alleles does not influence the risk of developing PE (Table 2). The analysis also indicated no association between PE and the C and T alleles. There was no significant correlation between these mutations and the occurrence of PE (P > 0.05). Examination of the +936C/T genotype revealed that the heterozygous CT genotype was more prevalent in both groups than in normal and PE women; however, there was no significant difference in the incidence of PE across these three genotypes (P > 0.05).
| Genotypes | Control Group | PE Group | Odds Ratio (Confidence Interval) | P-Value |
|---|---|---|---|---|
| SNP-634C/G | ||||
| CC | 54 (40.3) | 44 (32.8) | 0.724 (0.440 - 1.193) | 0.205 |
| CG | 54 (40.3) | 59 (44.0) | 1.165 (0.717 - 1.893) | 0.536 |
| GG | 26 (19.4) | 31 (23.1) | 1.250 (0.695 - 2.248) | 0.455 |
| CG + GG | 80 (59.7) | 90 (67.2) | 1.381 (0.838 - 2.274) | 0.205 |
| SNP + 936C/T | ||||
| CC | 52 (38.8) | 52 (38.8) | 1.105 (0.617 - 1.693) | 1 |
| CT | 70 (52.2) | 65 (48.5) | 0.861 (0.533 - 1.391) | 0.541 |
| TT | 12 (9.0) | 17 (12.7) | 1.477 (0.676 - 3.227) | 0.326 |
| CT + TT | 82 (61.2) | 82 (61.2) | 1.000 (0.612 - 1.635) | 1 |
Evaluation of Genotypes Related to -634C/G and +936C/T Polymorphisms a
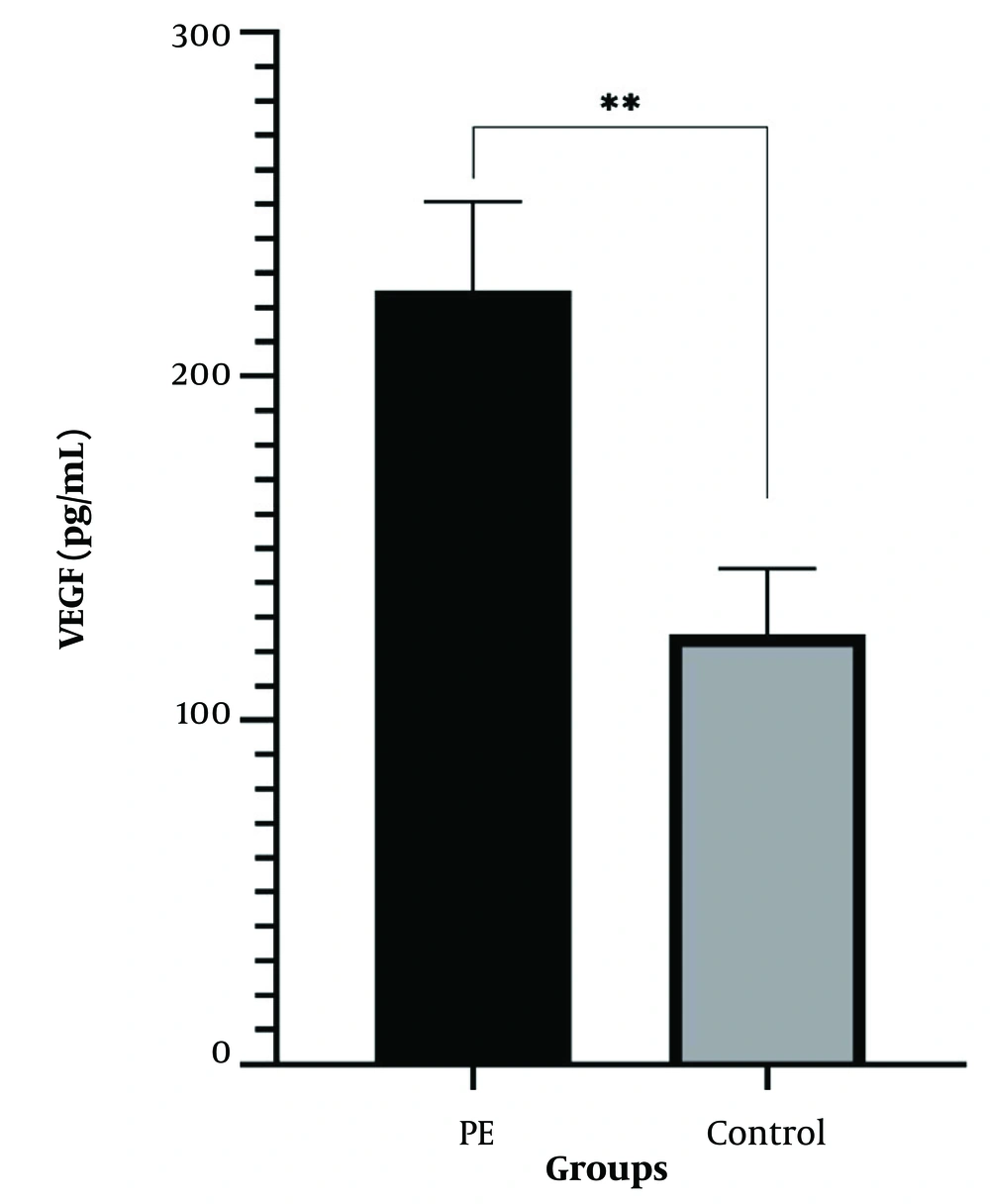
Table 3 illustrates the relationship between the +936C/T and -634C/G genotypes and serum VEGF levels in women with PE and the control group. The average concentration of serum VEGF in women with PE was recorded at 141.9 pg/ml, compared to 61.5 pg/ml in the normal group (Figure 1), demonstrating a significant statistical difference in the concentration of this factor between the two groups (P ≤ 0.001). In the group of patients, serum VEGF levels were significantly higher than in the normal group, suggesting that VEGF levels are elevated in these patients (P ≤ 0.05).
| Variables | Genotypes | P-Value | ||
|---|---|---|---|---|
| CC | CT | TT | ||
| VEGF with SNP +936 | ||||
| All Subjects | 141.4 ± 23.03 | 108.3 ± 17.59 | 126.4 ± 39.4 | 0.381 |
| Control | 104.2 ± 20.43 | 84.4 ± 17.7 | 20.6 ± 5.84 | 0.052 |
| PE | 174.4 ± 39.02 | 135.3 ± 31.35 | 220.4 ± 59.11 | 0.124 |
| VEGF with SNP-634 | CC | CG | GG | |
| All Subjects | 118.7 ± 21.56 | 122.2 ± 20.75 | 132.3 ± 29.56 | 0.841 |
| Control | 102.8 ± 23.44 | 85.9 ± 21.57 | 62.6 ± 14.17 | 0.838 |
| PE | 134.2 ± 36.06 | 155.5 ± 33.87 | 217.5 ± 58.09 | 0.232 |
Relationship Between +936C/T and -634C/G Genotypes and Serum VEGF Levels in Women with PE and Control Group a
5. Discussion
This study aimed to explore the polymorphisms of the VEGF gene in pregnant women with PE and found that, despite a significant increase in serum VEGF concentrations in these women, the -634C/G and +936C/T polymorphisms of the VEGF gene do not seem to be associated with the onset of PE.
Screening for PE using maternal factors and genomic variations is preferred over other methods, such as taking a medical history (14). In this research, maternal age and BMI, gestational age, infant weight, and hemoglobin concentration were identified as risk factors for PE. Similar to other studies, the mean age of the case group was significantly different from that of the control group, suggesting that the risk of PE increases with age (15). Another study associated an average age of over 45 with PE (16). It was also discovered that a lower gestational age at delivery in a previous pregnancy increases the risk of PE in subsequent pregnancies, serving as a strong predictor of the disease (17). However, another study found no correlation between BMI and PE (18).
Additionally, research using a national multicenter perinatal database indicated that advanced maternal age (≥ 45) correlates with a higher risk of adverse birth outcomes, particularly maternal complications such as PE (19). Given that the association of PE with ages over 45 has been established in various studies (16), our research suggests that the risk of PE may rise with advancing age. Echoing our findings, Sheen et al. found that older women had a higher likelihood of developing PE (20).
Therefore, considering this study included only women aged under 35 years, it can be inferred that the risk of PE increases with age across any maternal age range. This research also found that an increasing body mass index (BMI) heightens the risk of PE, aligning with findings from other studies (21, 22). A two-year cohort study on the impact of maternal pre-pregnancy BMI and gestational weight gain on PE risk demonstrated a link between being overweight and excessive weight gain with a heightened risk of PE (23). Similarly, another study identified being overweight and obese during pregnancy as risk factors for PE (24). Additionally, recent research evaluating the effect of maternal weight on maternal and perinatal outcomes suggested that gestational weight gain influences these outcomes and that pre-gestational BMI serves as a predictor for PE (25). Moreover, another study indicated no significant difference in hemoglobin levels between the control group and the PE group (26). While one study observed a higher occurrence of PE in summer (27), this study found no significant association between the season of occurrence and PE. However, recent research has highlighted that increased average temperatures pose a risk for pregnant women with PE, with such temperature rises being more prevalent in summer (28).
The imbalance between pro-angiogenic and anti-angiogenic factors in the mother's bloodstream is believed to influence the growth of normal endothelial function, which is a key characteristic of PE. Early research demonstrated that high levels of sFlt-1 expression induced by an adenovirus vector in rats could lead to gestational hypertension and renal proteinuria (27). Recent human genetic studies have indicated that genetic factors contribute to PE, yet the precise genetic pattern remains undefined. In this study, we aimed to establish the connection between the VEGF gene and the incidence of PE. The involvement of VEGF in PE warrants further exploration. Several reports have identified an elevation in serum VEGF levels in women with PE (26, 29). Our findings indicate an increase in VEGF levels in women with PE compared to the normal group, while another study found decreased VEGF levels in women with PE; this discrepancy might result from genetic differences across the studied populations or variations in the study cohorts (30).
Research has shown that in women with PE, an increase in the blood levels of a VEGF antagonist known as Sflt-1 reduces serum VEGF levels, whereas the level of this VEGF antagonist did not change in healthy pregnant women (30). Furthermore, one study discovered that the Sflt-1/placental growth factor (PlGF) ratio serves as an effective predictor of adverse maternofetal outcomes (AMFO) in confirmed cases of early-onset PE, potentially enhancing the treatment process by anticipating adverse effects (31). Given that most research has focused on the VEGF gene sequence in mothers, it is recommended that future studies investigate VEGF levels in the umbilical cords of infants as well as the polymorphism of this gene in neonates.
One study's results indicated an association between the frequency of the +936T allele and an increased susceptibility to PE (32). However, our study did not find a significant correlation between the incidence of PE and this single nucleotide polymorphism (SNP), a discrepancy that may be attributed to genetic differences. Given that our research did not focus on a single ethnic group but instead encompassed a diverse population, we recommend conducting further studies on specific Iranian ethnic groups, such as Lur, Kurd, Turk, Arab, etc., to explore the impact of racial factors on this issue.
In our study, no significant correlation was observed between the incidence of PE and the -634G/C and +936C/T polymorphisms. This finding aligns with another study that examined three SNPs of the VEGF gene (-2578C/A, -634G/C, and +936C/T) using the RFLP-PCR method, which also concluded that these SNPs do not have a relationship with PE (11). A limitation common to both studies was the reliance on the RFLP-PCR technique. Thus, we suggest that future research employ sequencing methods for a more detailed investigation of SNPs.
In another study, the allele frequency of three VEGF gene SNPs, including -460C/T, +405C/G, and +936C/T, showed no significant association with the incidence of endometriosis (33). In our study, an increase in VEGF levels in the sera of patients with PE was observed; however, there was no significant relationship between the incidence of PE and the +936C/T polymorphism.
5.1. Conclusions
In conclusion, managing weight through proper diet and regular exercise is effective in preventing PE in pregnant women. A decrease in hemoglobin levels may also contribute to the disease's severity. Given that serum VEGF levels were higher in the patient group compared to the normal group, this factor could serve as a predictive and diagnostic marker for the incidence of PE during pregnancy.