1. Background
Noninvasive prenatal testing (NIPT) serves as a screening technique to determine the risk of a fetus being born with chromosomal abnormalities such as trisomy 21, 18, and 13. It analyzes small DNA fragments circulating in the blood of pregnant women, known as cell-free DNA (cfDNA), which are not within cells and are free-floating (1). Consequently, cell-free fetal DNA ) cffDNA ( is considered a potentially valuable source of fetal genetic material for NIPT. The global adoption of NIPT for chromosomal aneuploidy screening has increased as pregnant women seek safer prenatal screening options (2).
CffDNA has emerged as a promising molecular biomarker utilized in various aspects of obstetrical research, particularly in complex pregnancies and prenatal diagnosis (3). The proportion of circulating cffDNA obtained from the fetoplacental unit, known as fetal fraction (FF), correlates with the reliability and success of test interpretation. Lower FF levels are sometimes associated with certain fetal disorders, leading to noninformative results (4).
While maternal age and other factors for aneuploidy may influence the performance of prenatal screening methods using cfDNA, there was no significant trend observed between maternal age and FF, particularly in groups with specific chromosomal abnormalities (5). However, aside from gestational age and maternal age, this study suggested that body mass index (BMI) could be associated with FF levels in different trisomic pregnancies undergoing NIPT. BMI, calculated using weight and height, may provide more descriptive information about maternal physiological conditions than weight alone (3).
If the levels of these DNA fragments fall below a certain threshold, the value of the cell-free DNA test diminishes.
2. Objectives
The aim of our study is to investigate the maternal and fetal factors affecting fetal fraction to determine whether NIPT should take precedence over other maternal screening tests or if influencing factors make the results of cfDNA less reliable, thereby suggesting alternative diagnostic tests.
3. Methods
Our research involved 1150 patients referred for non-invasive prenatal testing (NIPT) for various reasons, such as maternal age over 35, high-risk screening tests in the first or second trimester, history of trisomy in previous pregnancies, or patient request. Patients completed a questionnaire with variables including maternal and fetal age, BMI, smoking status, multiple pregnancies, and medication use such as Heparin or enoxaparin. NIPT was performed on blood samples using the Ion Proton platform by Premaitha of the UK. Results, including fetal sex, risk of trisomies, fetal fraction, and abnormal sex chromosomes, were entered into IBM SPSS 27 for analysis. The relationship between fetal fraction percentage and other variables was examined.
4. Results
A total of 1 150 cases underwent NIPT for this study. Maternal ages ranged from 13 to 48 years (Table 1), with BMIs ranging from 15 to 70. Gestational ages varied from 10 to 27 weeks. Of these cases, 96.4% (1 109) involved singleton pregnancies, while 3.6% (41) were twin pregnancies. In 88.9% of cases, pregnancies were spontaneous, 9.2% were via IVF, and 1.2% were via IUI. Fetal sex distribution was 45.9% female and 54.1% male. Fetal fraction ranged from 4% to 20.7%, with a mean of 11.07.
| Age | No. (%) | Cumulative Percent |
|---|---|---|
| 13 - 25 | 142 (12.3) | 12.4 |
| 26 - 35 | 509 (44.3) | 56.5 |
| 36 - 45 | 485 (42.2) | 98.8 |
| 45 < | 14 (1.2) | 100.0 |
| Total system | 1150 (100) |
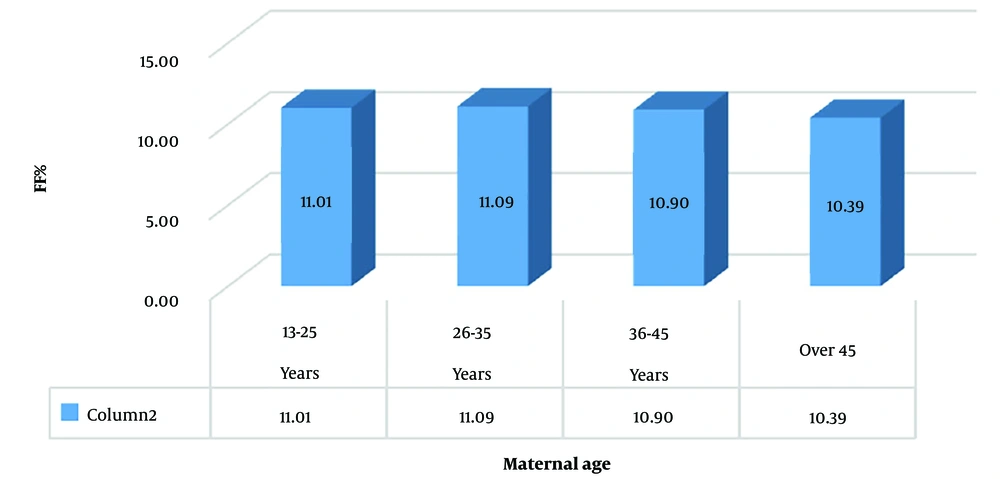
Among patients aged 13 - 25 years, comprising 12.3% of cases, the mean fetal fraction was 11.01% ± 2.3. The maximum was 20.3%, and the minimum was 4.46%. In the 26 - 35 age group (44% of cases, 506 patients), the mean fetal fraction was 11.09% ± 2.29, with a maximum of 20.7% and a minimum of 4.47%. For patients aged 36 - 45 (42.3% of cases), the mean fetal fraction was 10.9% ± 2.25, with a maximum of 20.33% and a minimum of 3.84%. Thirteen cases were over 45 years old, with a mean fetal fraction of 10.39% ± 2.85, a maximum of 15.9%, and a minimum of 5.6%.
While the mean fetal fraction did not significantly differ among the four age groups, the lowest mean fetal fraction was observed in the over 45 years old group (Figure 1).
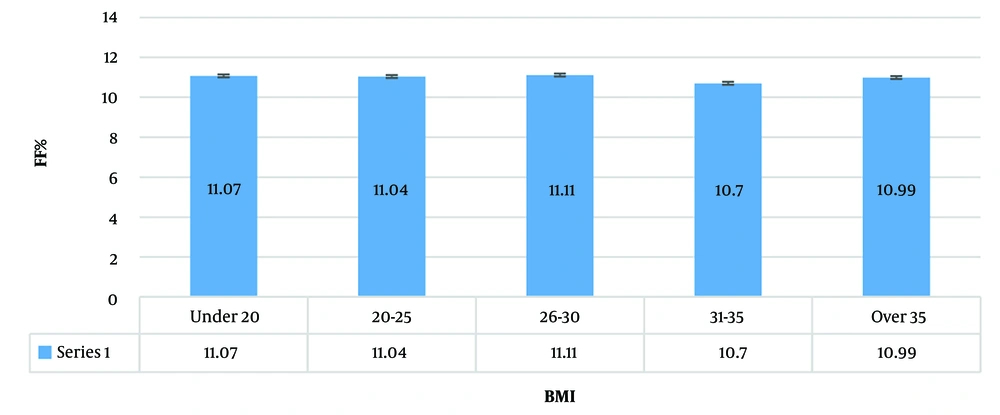
The BMI of 3.5 percent of the cases was under 20, 40.7% were between 25 to 30, and 7% of the subjects were over 35 (Figure 2).
The mean of BMI < 20 was 11.07% ± 2.09 (min: 5.09%, max = 15.37);
The mean of BMI 20 - 25 was 11.04% ± 2.31 (min: 4.7%, max: 20.7%);
The mean of BMI 26 - 30 was 11.11% ± 2.26 (min: 4.46%, max: 17.26%);
The mean of BMI 31 - 35 was 10.7% ± 2.3 (min: 3.8%, max: 17.94%);
The mean of BMI > 35 was 10.99% ± 2.1 (min: 4.47%, max: 17.26%).
Although the mean FF was lower in the over 35 BMI group, it is not statistically significant.
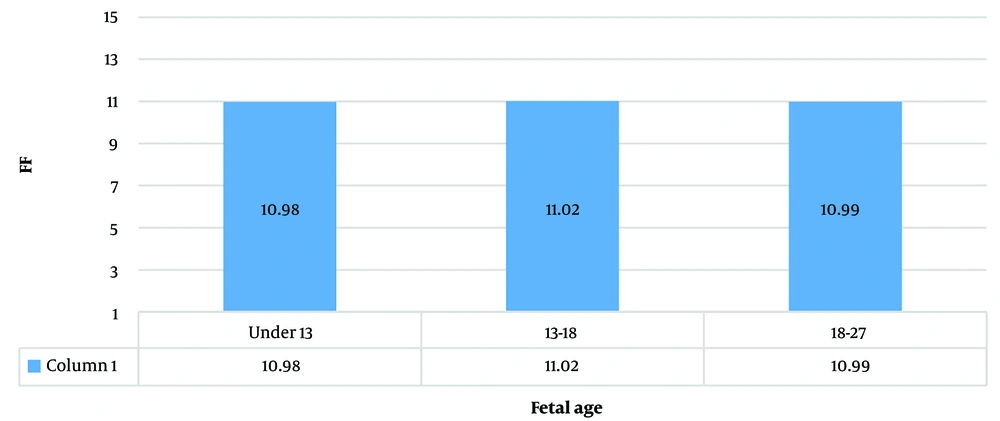
In 93.7% (1 066) of the cases, samples were taken under 18 weeks. 6.3% (72) were sampled between 18 to 27 weeks. Under 13 weeks, the mean FF was 10.98 ± 2.33 (min: 3.84, max: 20.7), between 13 - 18 weeks the mean FF was 11.02 ± 2.22 (min: 4.46, max: 20.33), and between 18 - 27 weeks the mean FF was 10.99 ± 2.3 (min: 4.47, max: 17.21) (Figure 3).
Because most of the cases were tested under 18 weeks (due to Iranian government law regarding abortion), the comparison was not very accurate, but between these groups, the difference was not statistically significant.
Of the 1 150 cases that underwent NIPT for this study, 1 109 (96.4%) were singleton pregnancies and 41 (3.6%) (Table 2) were twin pregnancies, which had a mean FF of 10.48. This is not significantly different from the mean FF in all cases (11.07).
| Variables | No. (%) | Mean FF | Cumulative Percent |
|---|---|---|---|
| Twin | 41 (3.6) | 10.48 | 100.0 |
| Single | 1109 (96.4) | 11.83 | |
| Total | 1150 (100) |
45.9% of fetuses were female with a mean FF of 10.98, and 54.1% were male with a mean FF of 11.02, which was not significantly different.
Trisomy 21 was recorded in 8 cases (0.7%), trisomy 18 in 2 cases (0.2%), and trisomy 13 in 1 case (0.1%) (Table 3).
| Variables | No. (%) | Cumulative Percent |
|---|---|---|
| Trisomy 13 | 1 (0.1) | 9.1 |
| Trisomy 18 | 2 (0.2) | 27.3 |
| Trisomy 21 | 8 (0.7) | 100.0 |
| Total | 11 (1.0) | |
| No trisomy | 1139 (99.0) | |
| Total | 1150 (100) |
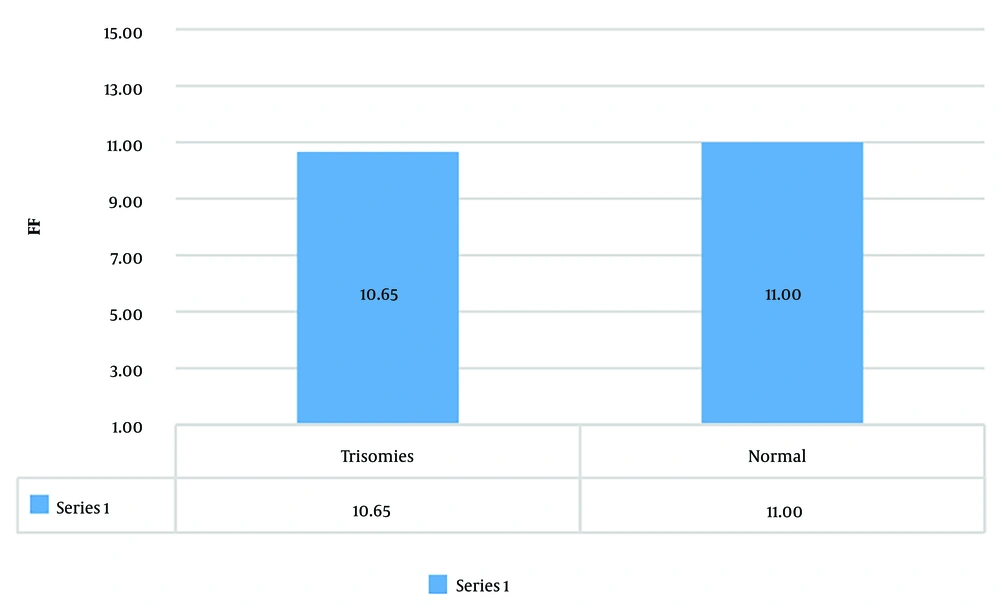
The mean FF in trisomies was 10.65 ± 2.08, with a Max of 14.66 and Min of 8.4, whereas in normal fetuses, the mean FF was 11 ± 2.28, with a Max of 20.7 and Min of 3.8 (Figure 4).
In 89.6% of cases, pregnancy was spontaneous, 9.2% were IVF, and 1.2% were IUI (Table 4).
| Variables | No. (%) | Cumulative Percent |
|---|---|---|
| Natural | 1030 (89.6) | 89.6 |
| IVF | 106 (9.2) | 98.8 |
| IUI | 14 (1.2) | 100.0 |
| Total | 1150 (100) |
The mean FF in the IVF group was 10.65, which was not significantly different.
5. Discussion
The factors considered in our study were BMI, maternal age, fetal age, maternal weight, and abnormal chromosomes. Regarding smoking and medication, the information provided by patients was incomplete and therefore removed from the analysis.
An analysis of 13 661 maternal plasmas for non-invasive prenatal screening by Hou et al. showed that an increase in BMI has a reverse effect on FF (3), but in our research, BMI had no significant effect. The study conducted by Burns and co-workers also indicated that obesity significantly decreased FF, consistent with findings on the association between anticoagulation therapy and maternal characteristics (6).
Scott et al. found that BMI and gestational age were significant factors, but our study did not replicate these results (7). Similarly, according to a study by Palomaki and co-workers, some fetal disorders such as trisomy were associated with systematically lower FF, leading to non-informative results (4). However, in our study, there was no significant difference in FF between trisomy and normal groups.
Hedriana et al.'s study showed that the contribution of individual FF from each fetus in DZ twins was on average 32% lower than in singletons (8). In twin pregnancies, the FF of cfDNA reduces with Black race, obesity, treatment with LMWH, ASA, and CHTN, and increases with GA. IVF and obesity are linked to an increase in the chance of cfDNA test failure (9). 3.6% of our cases were twin pregnancies, and we did not find a significant difference.
According to Creswell and co-workers, IVF and high BMI are highly implicated in reduced FF compared to fetal aneuploidy. However, repeating NIPT yields a result in more than 70% of cases (10), which contrasts with our study findings.
In a review article by Deng C, it was found that cffDNA in maternal plasma forms the basis of NIPS. Fetal fraction is crucial for NIPS quality control, and understanding FF variations is essential for accurate NIPS results. Laboratory staff and genetic counselors play a significant role in ensuring accurate NIPS results by considering fetal-placental characteristics, experimental factors, maternal characteristics, and calculation methods (11).
5.1. Conclusions
In this research, the data demonstrate the effects of our variables (age, sex, etc.) on the fetal fraction by conducting NIPS. We observe that maternal age has no significant correlation with the fetal fraction, while fetal age increases the fetal fraction, although insignificantly. BMI, pregnancy route, and fetal sex had no effect on FF. Despite NIPS being safer and yielding better results, other factors can influence the outcomes, making it crucial to consider NIPS as a screening test, and counseling should be provided clinically both before and after the test. Although our variables were not significant, the priority of the test remains unchanged. Physicians and consultants should be well-versed in the protocols and have a comprehensive understanding of the complexities of NIPS, particularly considering maternal conditions that might affect the results.