1. Background
Apoptosis is a process characterized by typical morphological features such as plasma membrane blistering, cell shrinkage, chromatin condensation, and fragmentation. It is a type of programmed cell death regulated by the Bcl-2 family and caspase enzymes (1, 2). Apoptosis occurs in multicellular organisms and, if inhibited, can lead to uncontrolled cell division and potential tumor formation. Disruptions in the apoptosis process are linked to cancer, autoimmune disorders, and neurodegenerative diseases (3).
Caspases, many of which are the primary mediators of programmed cell death including apoptosis, necroptosis, and pyroptosis, are enzymes belonging to a family of proteases. The name "caspase" derives from their function, featuring a unique cysteine protease activity where a cysteine in their active site acts as a nucleophile to cleave target proteins at aspartic acid residues. This family of enzymes includes fourteen members in mammals, eleven of which are human enzymes, playing crucial roles by breaking down specific substrates from the sequence H3N+…Asp
These enzymes are synthesized as inactive precursors known as procaspases. The proteolytic processing of procaspases yields a tetrameric active caspase enzyme composed of two repeating heterotypic subunits. Caspases are categorized into two groups based on substrate specificity and procaspase structure: Initiator and executioner. Initiator caspases (caspase-8 and 9) activate executioner caspases (caspase-3 and 7) in response to specific cell death signals, which then cleave various cellular proteins to drive apoptosis (6). Actin, vimentin, keratin, and cadherin serve as suitable substrates for caspase enzymes (7, 8).
Caspase-3, comprising two subunits of 17 and 12 kDa, is activated by caspase-8, caspase-9, and caspase-10, and subsequently activates caspase-6 and caspase-7.
Caspase-7, also known as "apoptosis-associated cysteine peptidase," is encoded by the CASP7 gene. This enzyme, in its precursor form, is activated by caspase-3, caspase-9, and caspase-10 (9). Caspases are involved in apoptosis through two main pathways: The extrinsic pathway, triggered by death receptors, and the intrinsic pathway, activated by cellular damage and stress.
Various factors influence the regulation, activation, and inhibition of caspases, and consequently apoptosis. These include the levels of intracellular calcium ions, the p53 molecule, adapter molecules, cytochrome c released due to the permeability of the mitochondrial outer membrane, Apaf-1 (apoptotic protease activating factor), and IAP (Inhibitor of Apoptosis Protein) (10-14).
Caspases are activated intrinsically due to cellular damage and stress, involving mitochondrial cooperation. Since platelets contain mitochondria, they can initiate the intrinsic or mitochondrial pathway of apoptosis. This process involves the permeabilization of the mitochondrial outer membrane and the transfer of cytochrome c, AIF (apoptosis-inducing factor), or Smac, leading to caspase-dependent or independent cytosolic signaling that activates caspase (15). During intrinsic activation, cytochrome c from mitochondria works together with caspase-9, Apaf-1, and ATP to activate caspase-3. Permeabilization of the mitochondrial outer membrane involves proteins from the Bcl-2 superfamily, which includes anti-apoptotic members (Bcl-2, Bcl-xL, Bcl-w) and pro-apoptotic members (Bid, Bad, Bak, Bax, and Bim). The presence of apoptotic stimuli promotes the accumulation and oligomerization of Bak/Bax in the mitochondrial outer membrane, forming a permeable transport pore (PTP) (16).
Although it is well understood that most nucleated cells can undergo caspase-dependent apoptosis, the functions of apoptosis regulatory molecules in anucleated cells like platelets remain largely unexplored.
2. Objectives
Osmotic stress affects not only cell morphology but also induces chemical changes within cells. This study aims to examine the impact of osmotic stress on the activity of intracellular apoptotic enzymes, specifically executioner caspases-3 and caspase-7 in platelets, which lack a caspase-independent apoptotic signaling pathway.
3. Methods
In this study, a bag of fresh platelets was obtained from the Iranian Blood Transfusion Organization. Platelets were separated from plasma by centrifugation at 3000 rpm for 10 minutes and washed with 0.9% sodium chloride, followed by three additional centrifugations as previously described. A suspension of platelets was then prepared.
Platelets were subjected to hypertonic osmotic stress (1.7% sodium chloride) and a non-stressed isotonic environment (0.9% sodium chloride) for durations of 15 minutes and 24 hours at 37°C. The Dr. Hielscher ultrasonic homogenizer was used to lyse the samples with 45 watts for 10 minutes on ice. The resulting lysate was then centrifuged at 2000 rpm for 10 minutes. Platelet counts were conducted before and after lysis using a Sysmex K 1000 cell counter (Japan) to confirm lysis.
Analytical tests were conducted on the lysates using the following methods:
- Immunoassay method: Caspase-3 levels were measured using an ELISA kit (Bioassay Technology Laboratory [BT LAB], China), employing wells coated with caspase-3 monoclonal antibodies. Each sample's absorbance was read in triplicate at a wavelength of 450 nm using a BioTek microplate reader (USA).
- Colorimetric method: Caspase-3/7 activity was assessed using a colorimetric kit (Kiazist Life Sciences, Iran). Enzyme present in each sample was exposed to a colorimetric substrate in triplicate, cleaving the substrate attached to a chromogen, resulting in a yellow color. This change was measured at a wavelength of 405 nm using a BioTek microplate reader (USA).
The data from each platelet sample were analyzed using SPSS software with a Two-Way ANOVA test, and significance was determined at a probability level below 0.05. Duncan and Tukey tests were also utilized to further analyze the data.
4. Results
The number of platelets in each sample, as counted by the Cell Counter device, is shown in Table 1.
| Row | Platelet Sample | Platelets Count Per Microliter of Sample |
|---|---|---|
| 1 | Platelet suspension before lysis | 375 × 103 |
| 2 | Platelet suspension after lysis | 20 × 103 |
4.1. Calculation of Caspase-3 Level
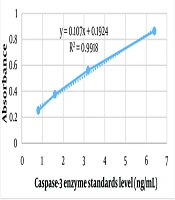
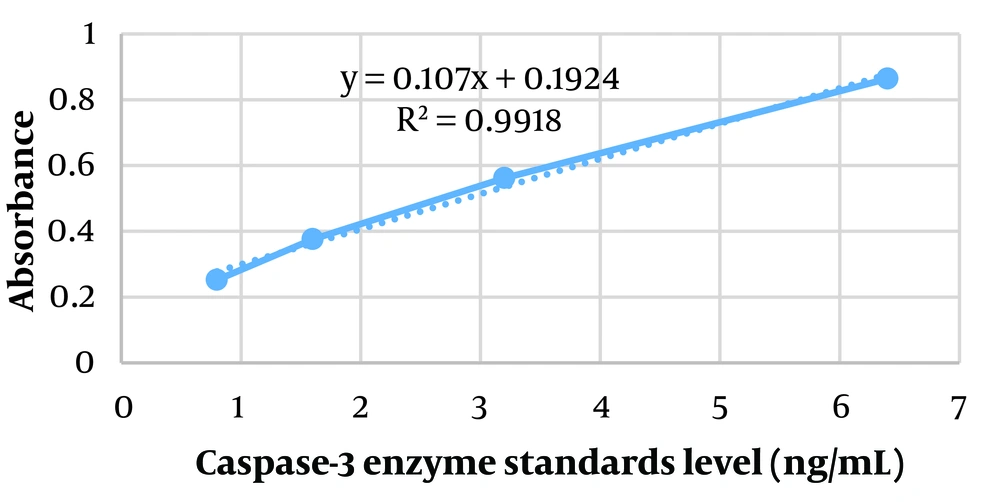
Using Excel software, the absorbance of the standards was read with a microplate reader at 450 nm, generating a standard curve (absorbance versus enzyme standards level), as shown in Figure 1. Based on the standard curve and the sample absorbances at 450 nm, the caspase-3 level in the samples was calculated in triplicate (Table 2). To facilitate data comparison, a histogram with Standard Error (SE) bars was prepared for each sample in triplicate (Figure 2). A statistically significant increase in caspase-3 levels was observed after hypertonic stress compared to the isotonic non-stress control environment, both at 15 minutes and 24 hours at 37°C. Additionally, extending the duration of stress from 15 minutes to 24 hours in a hypertonic environment significantly increased caspase-3 level (P-value < 0.05).
| Row | Samples | Absorbance of the Samples After Subtracting the Absorbance of the Blank | Caspase-3 Levels in the Samples, ng/mL |
|---|---|---|---|
| A | Blank | 0.09 | 0 |
| B | Standard 1 | 0.252 | 0.8 |
| C | Standard 2 | 0.376 | 1.6 |
| D | Standard 3 | 0.562 | 3.2 |
| E | Standard 4 | 0.864 | 6.4 |
| 1 | Isotonic lysate after 15 minutes (37℃) | 0.305 – 0.311 – 0.309 | 1.06 – 1.11 – 1.09 |
| 2 | Hypertonic lysate after 15 minutes (37℃) | 0.456 – 0.453 – 0.451 | 2.46 – 2.44 – 2.42 |
| 3 | Isotonic lysate after 24 hours (37℃) | 0.314 – 0.326 – 0.319 | 1.14 – 1.25 – 1.19 |
| 4 | Hypertonic lysate after 24 hours (37℃) | 0.638 – 0.648 – 0.642 | 4.17 – 4.26 – 4.21 |
A histogram comparing the mean caspase-3 level along with SE bars based on rows 1 to 4 of Table 2
4.2. Calculation of Caspase-3/7 Activity
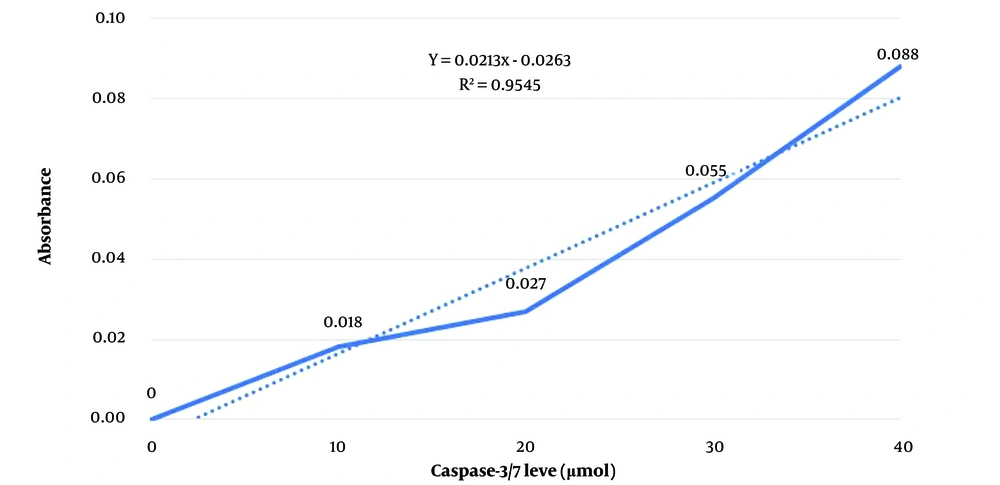
Using Excel software, the absorbance of enzyme standards in micromoles was read with a microplate reader at 450 nm to establish a standard curve (absorbance versus enzyme standards level), depicted in Figure 3.
The absorbance of samples subjected to stress in isotonic and hypertonic environments for 15 minutes and 24 hours at 37°C was measured at a wavelength of 405 nm. From the standard curve and sample absorbance measured in triplicate, caspase-3/7 levels were determined in µmol (Table 3).
| Samples | Repeat 1 | Repeat 2 | Repeat 3 | AverageCaspase-3/7 Level, µmol |
| Standard 0 | - | - | - | 0 |
| Standard 1 | 0.018 | - | - | 10 |
| Standard 2 | 0.027 | - | - | 20 |
| Standard 3 | 0.055 | - | - | 30 |
| Standard 4 | 0.088 | - | - | 40 |
| Isotonic lysate after 15 minutes (37℃) | 0.039 | 0.041 | 0.040 | 24.6 |
| Hypertonic lysate after 15 minutes (37℃) | 0.022 | 0.024 | 0.023 | 12.3 |
| Isotonic lysate after 24 hours (37℃) | 0.027 | 0.030 | 0.029 | 21.2 |
| Hypertonic lysate after 24 hours (37℃) | 0.021 | 0.019 | 0.023 | 15.5 |
Then, the obtained enzyme level, denoted as M, was input into the following formula to calculate the caspase-3/7 activity at T = 120 minutes for each environment. The sample dilution coefficient was set at 1.
Subsequently, the final result was multiplied by 1000 to convert the data from a decimal to an integer, expressing the unit of activity as nmol/min/L (Table 4).
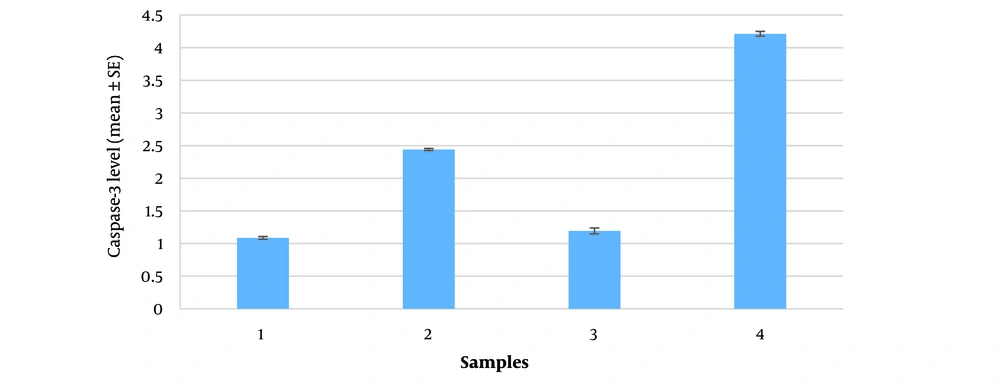
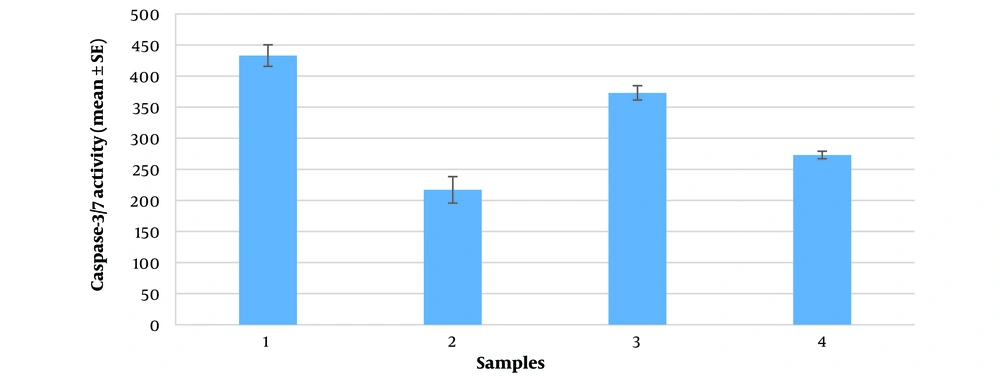
| Row | Samples | Repeat 1 | Repeat 2 | Repeat 3 | Average Caspase-3/7 Activity, nmol/min/L |
|---|---|---|---|---|---|
| 1 | Isotonic lysate after 15 minutes (37℃) | 416 | 457 | 427 | 433 |
| 2 | Hypertonic lysate after 15 minutes (37℃) | 195 | 246 | 211 | 217 |
| 3 | Isotonic lysate after 24 hours (37℃) | 357 | 382 | 381 | 373 |
| 4 | Hypertonic lysate after 24 hours (37℃) | 274 | 266 | 281 | 273 |
To facilitate a better comparison of the data, a histogram with the standard error (SE) was created for each sample in triplicate (Figure 4). There is a statistically significant decrease in caspase-3/7 activity after hypertonic stress compared to the non-stressed isotonic control environment, observed both at 15 minutes and 24 hours at 37°C. Notably, extending the duration of stress from 15 minutes to 24 hours in the hypertonic environment leads to an increase in caspase-3/7 activity (P-value < 0.05).
5. Discussion
Platelet counting data indicate that the ultrasound homogenizer successfully lysed up to 95% of the platelets, releasing their contents including caspase enzymes. In this study, exposure of platelets to abiotic stress, such as osmotic stress induced by a hypertonic environment, led to changes in the platelet membrane and mitochondria. This included the transfer of cytochrome c, which subsequently increased the level of caspase-3, an executioner caspase. Extending the duration of hypertonic stress to 24 hours approximately doubled the caspase-3 level. Despite this increase, as measured by the ELISA immunoassay method, the activity of caspase-3/7, measured using the colorimetric method, showed a decrease after both 15 minutes and 24 hours compared to non-stressed isotonic environments.
In protein and enzyme structures, besides the freely moving bulk water around them, two types of water are attached to the enzyme. Loosely bound water molecules are attached to each amino acid molecule at a ratio of 2.5 water molecules per amino acid, with their movement being only one hundredth that of the bulk water. Tightly bound water molecules constitute 1% of the loosely bound water molecules, with their movement being one millionth that of the bulk water around the enzyme (17). The presence of water molecules at the enzyme active site significantly impacts the interactions between the substrate and the active site, so reducing water concentration in hypertonic conditions can likely decrease caspase-3/7 activity.
Caspase-3 in anuclear cells likely originates from stable mRNAs or from remaining caspase proenzymes in the cytoplasm of platelets. Under osmotic stress, caspase-3 level increase significantly, as shown by its antigenic property binding to antibodies. ELISA immunoassay tests revealed high caspase-3 level (2.44 and 4.21 ng/mL after 15 minutes and 24 hours of hypertonic stress, respectively) compared to non-stressed conditions (1.08 and 1.19 ng/mL). However, this higher expression level coincides with a lower enzyme activity (217 and 273 nmol/min/L compared with 433 and 373 nmol/min/L in the same conditions mentioned above).
This study suggests that the decreased activity of executioner caspases, despite their increased levels, could be due to alterations in their three-dimensional structure caused by increased osmotic pressure. A reduction in water molecules bound to the enzyme could alter its spatial structure, reducing its activity. Optimal enzyme activity depends on several factors: Quantity and quality of the enzyme, adequate substrate, temperature, and pH. Interestingly, some enzymes retain activity even in organic environments (18). This research shows that the tonicity and osmotic pressure surrounding an enzyme in an aqueous medium also affect enzyme activity.
5.1. Conclusions
This study confirms the presence and activity of executioner caspase enzymes in platelets under non-stressed isotonic conditions. It demonstrates that caspase-3 level significantly increase after 15 minutes and 24 hours of hypertonic osmotic stress, with a statistically significant increase (P-value < 0.05) compared to non-stressed isotonic conditions. However, caspase-3/7 activity decreases under the same conditions, highlighting the complex effects of hypertonic stress on enzyme behavior. Extending stress from 15 minutes to 24 hours can increase both the level and activity of these enzymes, indicating that the hypertonic environment of 1.7% sodium chloride in an anuclear cell like a platelet raises caspase-3 level, but molecularly, this stress reduces caspases-3/7 activity due to the aforementioned reasons.