1. Background
Alzheimer's disease (AD) is a debilitating neurodegenerative disorder characterized by the formation of amyloid plaques, neuroinflammation, oxidative stress, and cell death. There is currently no definitive cure for Alzheimer's, and existing treatments can only slow the progression of the disease (1). Recently, herbal supplementation and exercise have gained attention as non-pharmacological approaches due to their anti-inflammatory and antioxidant polyphenolic compounds (2). Exercise training can enhance neurological health by increasing neurotrophic factors (3). Previous studies have indicated that melilotus extract can prevent oxidative damage by regulating the expression of genes such as peroxiredoxin 6 (Prdx6) and lysine acetyltransferase 2B (KAT2B). The antioxidant genes PRDX6 and KAT2B play roles in combating oxidative stress, and the clusterin gene encodes a protein that protects against oxidative stress. The gamma-secretase enzyme is involved in cell signaling pathways.
Reports have shown that the expression of these genes and enzymes is altered in brain tissue, particularly in the hippocampus of Alzheimer's patients. The antioxidant enzyme PRDX6 protects cells against oxidative damage by reducing peroxides. Decreased PRDX6 expression leads to increased oxidative stress and inflammation in AD. Lysine acetyltransferase 2B, a nuclear acetyltransferase, plays an important role in regulating the transcription of anti-inflammatory genes. Reduced KAT2B activity is associated with chronic inflammation (4). Strength training induces the secretion of neurotrophic factors such as nerve growth factor (NGF). Nerve growth factor is essential for the growth and maintenance of neurons, playing a significant role in preserving neural function. Pourrabi and Hossini have shown that androgens, aside from regulating sexual behavior, can exert a neuroprotective function in the brain (5).
Clusterin is a multifunctional glycoprotein that impacts several physiological and pathological conditions, including AD. Its role in fat transport, the immune system, and oxidative stress has been established. The relationship between clusterin and amyloid-beta is known to influence the accumulation and clearance of Aβ in AD and mediate Aβ toxicity. CLU is also an important genetic risk factor for late-onset AD, and changes in CLU expression at both mRNA and protein levels can affect cognitive function, memory, and brain structure (6).
Clusterin has been identified as a soluble cytoplasmic protein that protects cells by inhibiting lipid peroxidation (7). The beta-secretase enzyme is crucial in cell signaling pathways and proliferation, with changes in its activity linked to neurological diseases. Brain-Derived neurotrophic factor (BDNF) is an essential protein that supports neuron growth and survival, and decreased BDNF levels are associated with AD (8).
Sports training also activates cellular antioxidant defense mechanisms by inducing factors such as nuclear erythroid-related factor 2 (Nrf2). Nuclear erythroid-related factor is a transcription factor that plays a protective role against oxidative stress, which is one of the pathological mechanisms involved in AD (9). Resistance training stimulates and induces muscular adaptation, increasing neurotrophic factors and improving mitochondrial function (10, 11). There is growing interest in non-pharmacological therapies like herbal supplementation and structured exercise regimes (12).
2. Objectives
This study aimed to investigate the combined effect of resistance training exercises with weights and supplementation of melilotus officinalis on the expression of antioxidant and anti-inflammatory genes; beta-secretase enzyme, clusterin, BDNF, NGF, Nrf2, PRDX6, and KAT2B in the hippocampal tissue of an Alzheimer's model rat.
3. Methods
The statistical population of this research consisted of Wistar male rats. The sample comprised 55 male rats (weighing 180 - 220 grams; aged 8 - 10 weeks), obtained from the Pasteur Institute of Iran. These rats were housed in polycarbonate cages under standard conditions (12-hour light-dark cycle, temperature maintained at 22 - 24°C, relative humidity of 55 - 60%), with free access to water and food (13).
3.1. Resistance Training Protocol
The rats in the resistance training groups underwent training sessions for one week (five sessions) to familiarize themselves with climbing a ladder. All ethical principles were observed during the execution phases. The resistance training lasted for eight weeks, with three sessions per week on a ladder consisting of 26 steps with a 4-centimeter distance between steps. Each training session for the rats comprised 4 sets (14).
3.2 Preparation of Melilotus Extract
Fresh melilotus leaves were separated and powdered. The obtained powder was mixed in a 10:1 ratio by weight with 80-degree alcohol. Daily, 1500 mg of the extract powder mixed with 6 mL of normal saline was injected intraperitoneally into each rat at a concentration of 30 IU (International Units) of the solution (15). After 12 hours of fasting, AD was induced using a single dose of 10 milligrams per kilogram of trimethyltin chloride (TMT) via intraperitoneal injection (16). Memory and learning were assessed using the Shuttle Box test on a group of afflicted rats compared to a healthy control group (17). Following confirmation of AD induction in rats, they were randomly divided into groups: (1) AD control (A), (2) AD + melilotus extract supplement (AM), (3) AD + resistance exercise (AR), and (4) AD + resistance exercise + melilotus extract (ARM). Additionally, 11 healthy rats were placed in a control group (HC) to examine the effects of Alzheimer's induction on research variables in healthy controls. RNA extraction from the total cell contents was performed using a column RNA extraction kit. For each primer, the PCR efficiency was measured.
Clusterin, beta-secretase enzyme, BDNF, NGF, Nrf2, PRDX6, and KAT2B genes were used to investigate the changes in testicular tissue of rats and inflammatory and stress factors. After 48 hours of the last training session and 12 hours of fasting, rats were anesthetized using ketamine (50 mg/kg) and xylazine (15 mg/kg). The upper part of the animal's skull was shaved, and the skull tissue was cut with a cutter and scissors. Brain and hippocampus tissues were separated by experts. After sampling, a thin slice of the tissue was prepared and placed in a sterile envelope (Figure 1).
A two-way analysis of variance was used to compare the differences between the averages of the experimental groups and the control group. Tukey's post hoc test was used when significance was detected (P ≤ 0.05).
4. Results
The expression of the secretase beta gene was significantly decreased in the AMR group compared to the A group (P = 0.020). There was no significant difference between the HC group and the AMR group. The Tukey test did not show a significant difference in the AM (P = 1.000) and AR groups (ŋ = 0.518, P = 0.025, F = 3.121) (Figure 2).
The difference between groups in beta gene expression in hippocampal tissue determined using two-way analysis of variance and Tukey's post hoc test (P ≤ 0.05). #, A significant increase is observed in the Alzheimer's group compared to the Alzheimer + resistance training group, Alzheimer + melilotus extract group, and Alzheimer + melilotus extract + resistance training group.
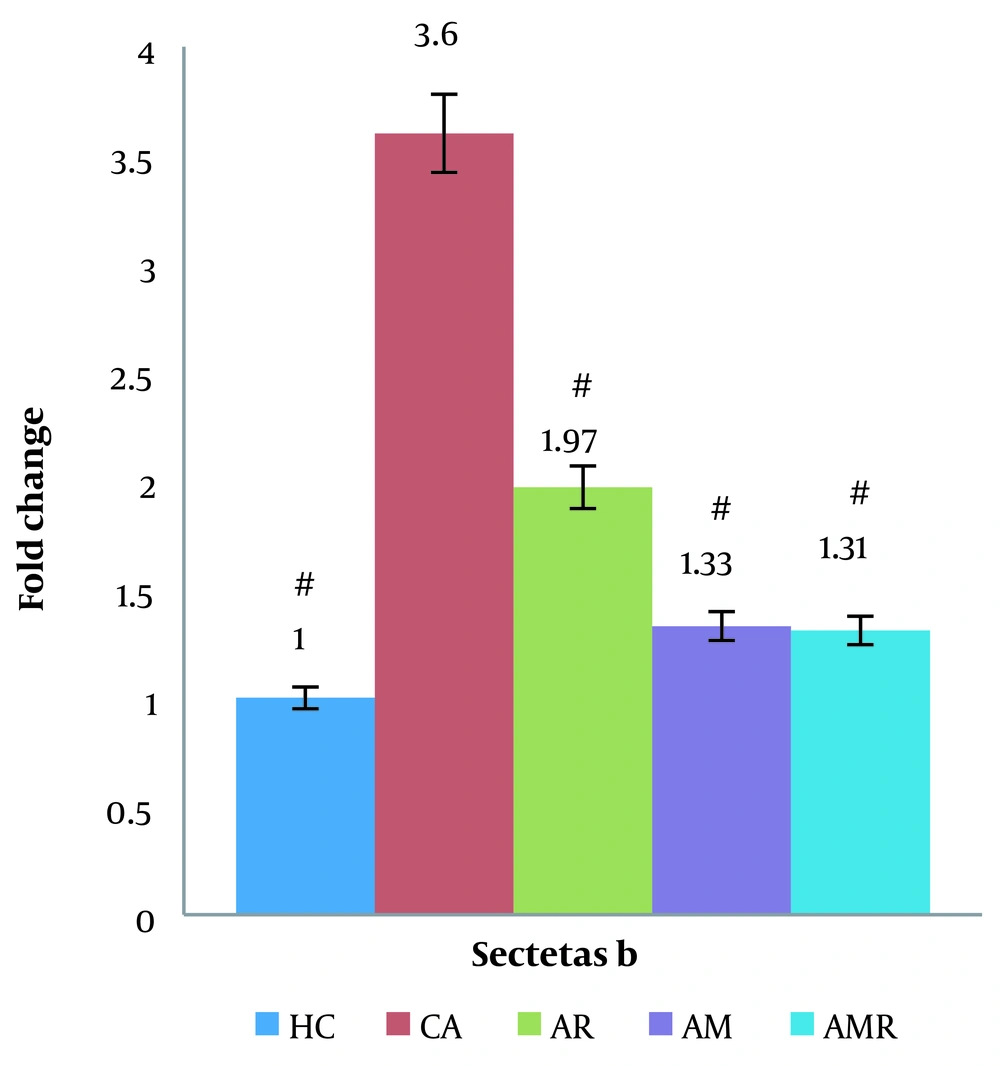
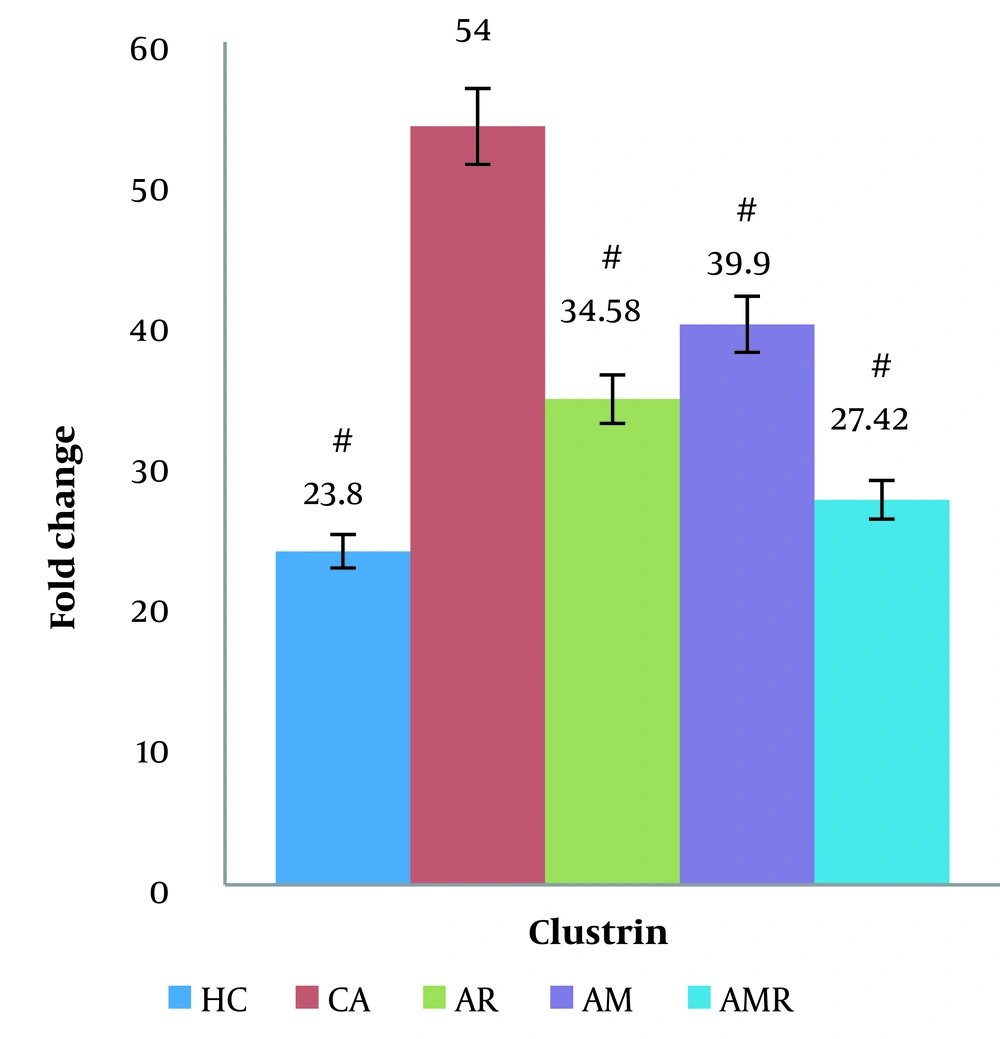
Based on the results obtained from the two-way ANOVA, the level of secretase beta gene expression in the AD group significantly increased compared to the control group (P < 0.05). The expression level of the secretase beta gene increased less in the resistance training group and the AMR group. The expression of the clusterin gene was significantly decreased in the AMR group compared to the A group (P = 0.000). There was no significant difference between the HC group and the AMR group. The Tukey test did not show a significant difference in the AM (P = 1.000) and AR groups (ŋ = 0.149, P = 0.009, F = 4.259) (Figure 3).
The difference between groups in clusterin gene expression in testicular tissue determined using two-way analysis of variance and Tukey's post hoc test (P ≤ 0.05). #, A significant increase is observed in the Alzheimer's group compared to the Healthy control group, Alzheimer's group, Alzheimer + resistance training group, Alzheimer + melilotus extract group, and Alzheimer + melilotus extract + resistance training group.
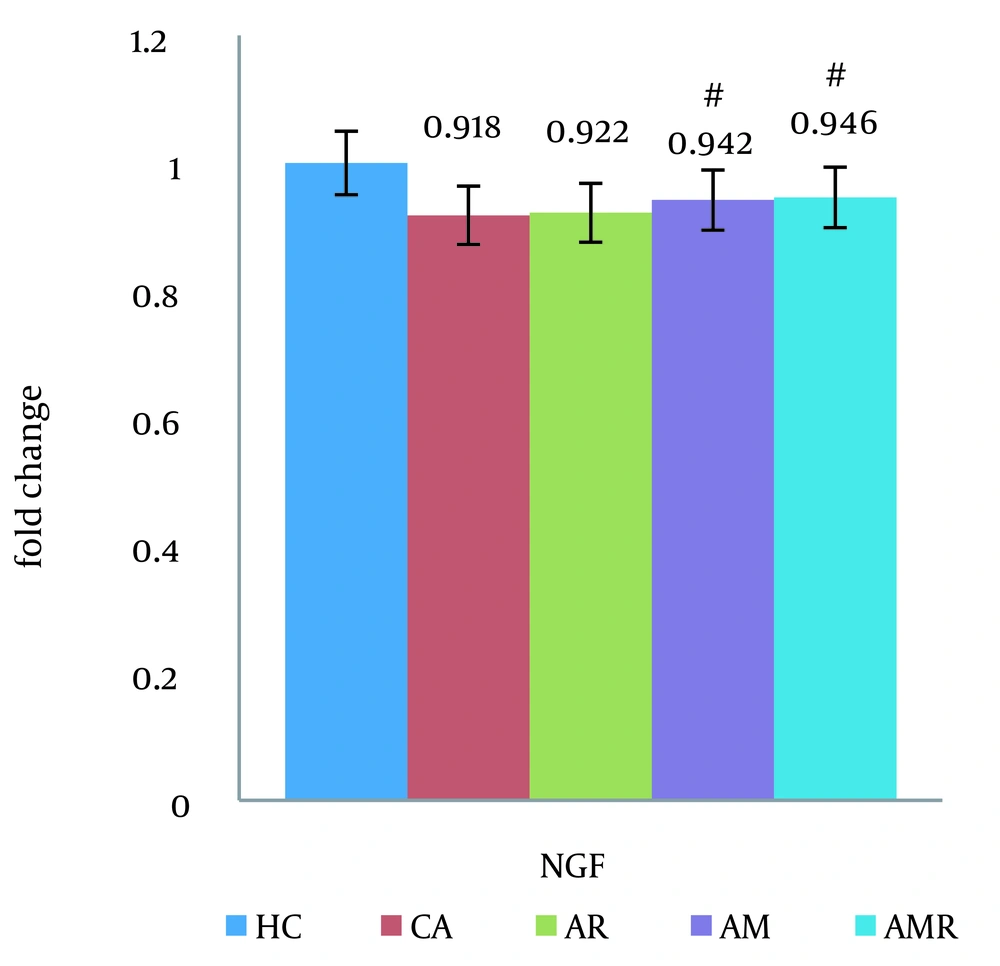
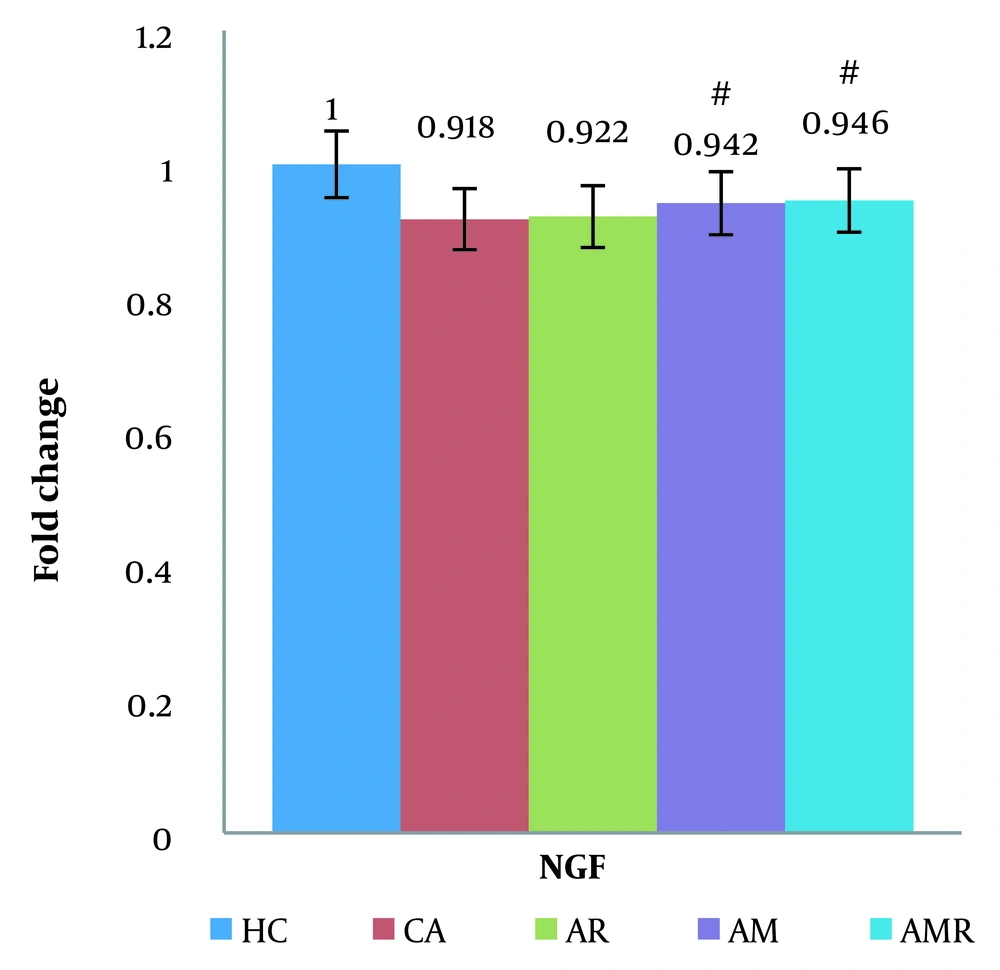
Based on the results of the two-way analysis of variance, the mRNA BDNF difference between the A group and the AMR group (ŋ = 0.425, P = 0.062, F = 4.541) was significant. The expression of the NGF gene was significantly increased in the AMR group compared to the A group (P=0.000) (Figure 4). There was no significant difference between the HC group and the AMR group. The Tukey test did not show a significant difference in the AM (P = 1.000) and AR groups (ŋ = 0.838, P = 0.018, F = 4.170).
The difference between groups in nerve growth factor gene expression in hippocampal tissue was determined using two-way analysis of variance and Tukey's post hoc test (P ≤ 0.05). #, A significant decrease is observed in the Alzheimer's group compared to the Alzheimer's + melilotus extract + resistance training group.
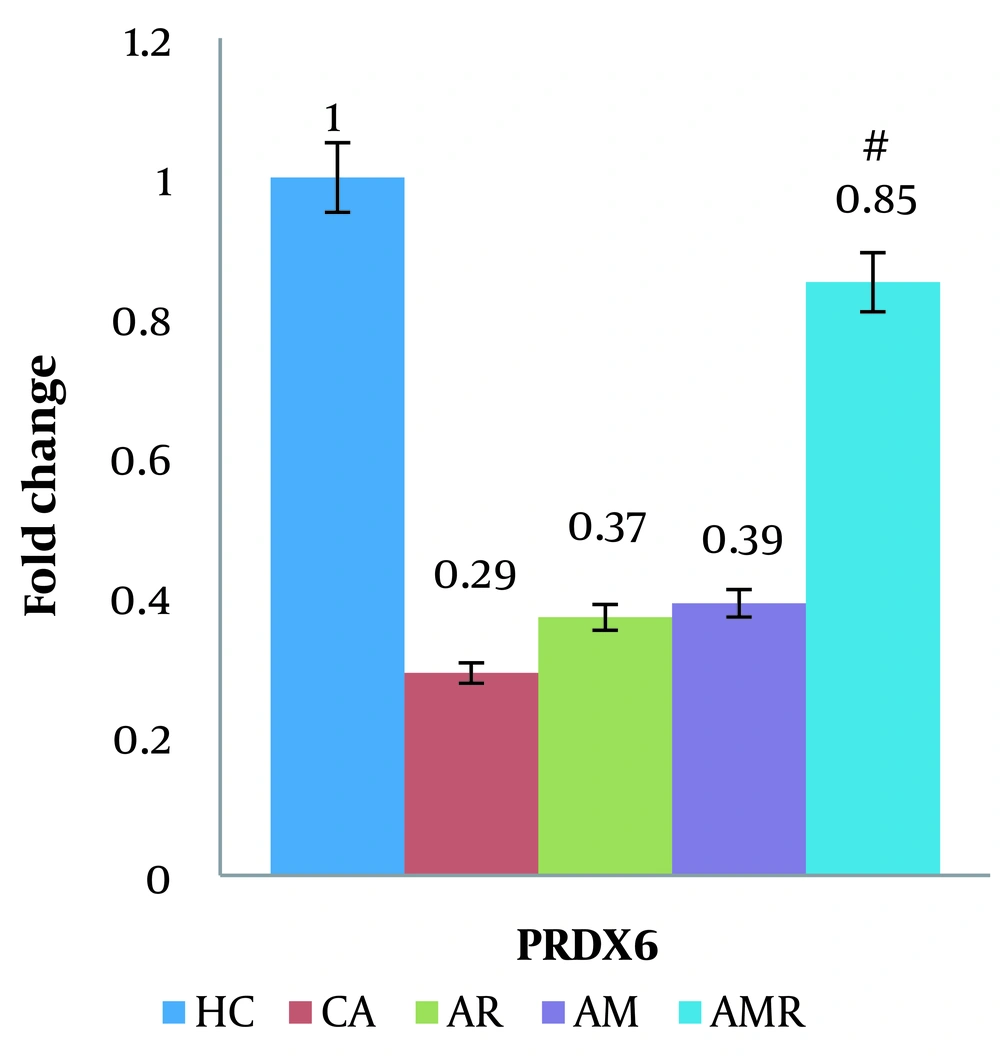
The expression of the PRDX6 gene was significantly increased in the AMR group compared to the A group (P = 0.001). There was no significant difference between the HC group and the AMR group. The Tukey test did not show a significant difference in the AM (P = 1.000) and AR groups (ŋ = 0.832, P = 0.024, F = 3.142) (Figure 5).
The difference between groups in PRDX6 mRNA gene expression in hippocampal tissue was determined using two-way analysis of variance and Tukey's post hoc test (P ≤ 0.05). #, A significant increase is observed in the Alzheimer's + melilotus extract + resistance training group compared to the Alzheimer's group.
Based on the results of the two-way analysis of variance, the mRNA KAT2B difference between the A group and the AR group (ŋ = 0.321, P = 0.054, F = 3.211) was significant (Figure 6).
The difference between groups in lysine acetyltransferase 2B mRNA gene expression in hippocampal tissue was determined using two-way analysis of variance and Tukey's post hoc test (P ≤ 0.05). #, A significant decrease is observed in the Alzheimer's group compared to the Alzheimer's + resistance training group.
5. Discussion
The results of this research indicated that the gene expression levels of clusterin in testicular tissue and beta-secretase in hippocampal tissue decreased, while the expression levels of BDNF in hippocampal tissue increased. Additionally, the expression of NGF, PRDX6, and KAT2B in hippocampal tissue also increased in the exercise and melilotus groups. These findings align with the studies conducted by He et al., Kishi et al., Meng et al., and Jaberi and Fahnestock (18-21), but contradict the results of Malik et al. and Gray et al. (22, 23).
It appears that exercise can reduce the level of Aβ in AD, and the reduction in beta-secretase levels leads to the breakdown of amyloid precursor proteins. Alizadeh et al. also demonstrated that regular physical activity and a healthy lifestyle are crucial for CNS adaptation in the hippocampus (24). Changes in the clusterin gene can impact brain biochemical pathways, and resistance exercise has been shown to enhance brain pathways that produce neurotrophic factors.
Melilotus extract provides antioxidant compounds, which reduce oxidative stress and clusterin inflammation through cellular protective signaling cascades (18). Various disease states, such as neurodegeneration, are associated with clusterin secretion and its cellular protective or anti-apoptotic functions. The increased clusterin level observed in the presence of toxic protein aggregates is a common pathological feature. Foster et al. reported an increase in clusterin in hypoxia-ischemia mice, implicating its role in underlying pathogenic processes and AD. Thus, in the training groups, these conditions were not present to increase clusterin levels, suggesting that training effectively improved blood circulation (6).
Melilotus contains various bioactive compounds, including coumarins, flavonoids, and phenolic acids, which exhibit anti-inflammatory, antioxidant, and neuroprotective properties. These compounds likely improve brain function by reducing nerve damage, increasing the expression and activity of antioxidant enzymes, lowering inflammatory marker levels, and enhancing apoptosis. Additionally, melilotus strengthens the regeneration of nerve cells in the hippocampus and cerebral cortex, prevents beta-amyloid plaque deposition and tau protein hyperphosphorylation, and activates the Nrf2 and Akt signaling pathways, as observed in this research.
The reduction of BDNF is probably related to the dose and duration of melilotus extract administration, the type and intensity of resistance training, and possibly the excessive muscle stress caused by reduced BDNF levels. Brain-Derived neurotrophic factor likely activates intracellular signaling pathways, stimulates dendritic growth, and promotes synaptic connections and neurogenesis in brain tissue by binding to TrkB receptors on neurons. Another possible mechanism is that resistance training, through mechanical muscle stretching and increased blood flow to the brain tissue, has led to nerve stimulation and the release of nerve growth factors and BDNF. It is also possible that the phenolic and antioxidant compounds in melilotus extract did not effectively induce the intracellular signaling pathways needed to synergize BDNF gene expression (25, 26).
Regarding NGF neurotrophin levels, there is evidence that dysregulation of neurotrophins affects cholinergic synapses and synaptic plasticity. Therefore, exercise and melilotus consumption may have contributed to the temporal regulation of NGF neurotrophins. Meng et al. reported that blood flow, hippocampus volume, and neurogenesis improvement were increased in AD patients who had long-term exercise interventions, which aligns with the findings of this research. Compared to drugs, exercise has fewer side effects (20). Strength training may impact vascular physiology, hippocampal volume, and neurogenesis. For the early stages of AD and the overall prevention of dementia, exercise appears to be an effective treatment and preventive measure (27).
Melilotus extract contains antioxidant compounds that can activate the Nrf2 signaling pathway. Activation of Nrf2 leads to increased expression of antioxidant genes, which can protect cells from oxidative damage. Hase et al. confirmed the activation of the Nrf2/ARE antioxidant pathway by investigating the potential neuroprotective mechanisms of plant extracts. Studies on long-term strength training have shown the activation of SIRT1 and Nrf2 pathways in brain tissue. Strength training induces the Nrf2 pathway by causing metabolic stress (28). Therefore, melilotus plant extract combined with strength training has a synergistic effect on activating Nrf2 in Alzheimer's rats. This synergistic effect may reduce oxidative and inflammatory damage in the brain by regulating the body's antioxidant defense system, and the combination of plant extracts and exercise provides a useful strategy to combat Alzheimer's symptoms in an animal model (29).
However, Gray et al. provided conflicting evidence about the effects of melilotus extract and exercise training on Nrf2 signaling in AD models, finding no significant effect on the expression of Nrf2 target genes in Alzheimer's rats (23). It should be noted that some research used older rats and endurance training methods (19).
Peroxiredoxin 6 is an antioxidant that plays a critical role in protecting cells from oxidative stress, while KAT2B is an acetyltransferase enzyme involved in regulating gene transcription. Resistance training and melilotus extract increased the expression of PRDX6 and KAT2B in the hippocampus. The induction of these two proteins had a beneficial effect on tissue health by enhancing antioxidant defense and reducing inflammation. Resistance exercise induced epigenetic changes and upregulated KAT2B gene expression, along with increasing neurotrophic factors and PRDX6. Jaberi and Fahnestock have also confirmed these findings. Additionally, reducing inflammation, improving brain blood flow, and enhancing mitochondrial health have influenced the expression of these genes (21). Other possible factors include increasing the production of NGF and antioxidant enzymes, stimulating angiogenesis, improving cerebral blood flow and neuron nutrition, creating epigenetic changes, regulating gene expression through effects on DNA methylation and histones, and modulating the activity of signaling pathways involved in neuron proliferation (30).
However, De la Rosa et al. showed that endurance training in old rats had no significant effect on the expression of PRDX6 and KAT2B proteins in brain tissue. The difference may be due to the chronicity of the exercises, the advanced age of the mice, and the severity of the brain injuries (31). Improta-Caria et al. also reported that endurance training in aged rats does not affect the expression levels of PRDX6 and KAT2B in the cerebral cortex (32). The potential mechanisms of melilotus's effects on AD emphasize its anti-inflammatory and antioxidant properties (33). However, limitations such as the small number of research samples, lack of laboratory tools, inadequate animal facilities, and difficulties in obtaining kits and laboratory materials due to the general embargo on Iran may prevent the full display of the study's results, capabilities, and potential.
5.1. Conclusions
In conclusion, this study has demonstrated for the first time the effectiveness of simultaneous exercise and supplementation with melilotus extract as a novel mechanism for controlling, modulating, and potentially halting the progression of AD. Given the significant impact of these independent variables on the dependent variables, a new mechanism is proposed that is both cost-effective and free from side effects.