1. Background
Breast cancer is one of the most common cancers in women (1). For centuries, plants and their biologically active compounds have been used for their therapeutic properties in treating various ailments. Herbal products, which contain anticancer compounds such as alkaloids, saponins, terpenes, glycosides, and polyphenols, have been shown to have minimal side effects. Notably, nearly 50% of the chemotherapy drugs in use today are derived from plants (2). Essential oils, valuable substances produced as secondary metabolites in different plant organs (3), have lipophilic properties that allow them to penetrate cell membranes and enter cells (4). These herbal compounds are utilized for various purposes, have fewer side effects than synthetic drugs, and can inhibit the growth and proliferation of tumors (5, 6). In recent years, there has been extensive research on the potential of essential oils and their specific compounds to combat cancer (7, 8). Due to the complex compounds in essential oils, their effects on cells can vary (9). Some essential oils have been proven to have cytotoxic effects on cancer cells without harming normal cells (5, 10-12).
Hymenocrater longiflorus, locally known as "Soura halala," is a native species of Hymenocrater found in northwest Iran (13). Traditionally, the aerial parts of this plant are used to treat skin diseases. Studies have confirmed the antioxidant and antimicrobial activities of H. longiflorus Benth. essential oil (14). However, few studies have explored its anticancer properties; thus, the present study investigates the effects of different doses of H. longiflorus Benth. essential oil on MDA-MB-231 and MCF-7 cancer cell lines.
2. Objectives
We aimed to examine the cytotoxic activity of H. longiflorus Benth. essential oil against two human breast cancer cell lines, MDA-MB-231 and MCF-7. Additionally, we analyzed the type of cell death induced by using flow cytometry and fluorescent microscopy. The chemical composition of the essential oil was also investigated using GC-MS.
3. Methods
3.1. Plant Harvesting and Extract Preparation
In spring 2019, the aerial parts of H. longiflorus Benth. were collected from Dezli village, Marivan County, Kurdistan Province, Iran (latitude 35'18°N, longitude 46'55'11°E, altitude 1950 m). The plant was authenticated at the Research Center of Agricultural and Natural Resources in Sanandaj, Kurdistan Province, Iran (voucher specimen number: 725). After drying in the shade, the plant was ground into powder. Essential oil extraction from the plant samples was carried out for three hours using the hydrodistillation method with a Clevenger apparatus. The obtained essential oil was dehydrated with anhydrous sodium sulfate (Germany, Merck), transferred into a glass vial, and stored at 4°C in a refrigerator (15).
3.2. Gas Chromatography-mass Spectrometry (GC-MS) Analysis
GC/MS is an analytical technique that separates volatile compounds using gas chromatography (GC) and identifies them using a mass spectrometer (MS). In this study, an Agilent 6890 series GC with a 5973 network mass selective detector (5973N MSD) was utilized to analyze and identify the compounds in the essential oil. A capillary silica column measuring 50 meters in length, 0.25 millimeters in diameter, and 0.25 micrometers in thickness was employed. Helium gas with a purity of 99.999% was used as the carrier gas, flowing through the column at a consistent rate of 1 mL/min. The initial temperature of the GC column was set at 60°C and was gradually increased to 280°C. The temperature of the GC/MS injector was maintained at 280°C to ensure complete vaporization of the components before they entered the column. 1 microliter of the essential oil was introduced into the GC column. A high-energy electron beam (70 electron volts (eV)) was used to ionize the sample, forming radical cations. The mass-to-charge (m/z) ratio, a unique property of each molecule, was used for identification. The detector was set to identify ions with m/z ratios between 40 and 600. The acceleration voltage solver (AVS) was activated after a delay of 4 minutes. The identification of components was based on comparing their retention times and peak fragmentation patterns to those in the NIST/Wiley library spectra.
3.3. MTT Test
Fifteen thousand cells were uniformly cultured in each well of a 96-well plate, each containing 200 microliters of growth medium. After the cells attached to the well surfaces and assumed a typical form, they were incubated with varying concentrations of essential oil for 48 to 72 hours. The incubation was conducted at a temperature of 37°C and a CO2 concentration of 5%. The culture medium was then removed from each well and the wells were washed with PBS. Subsequently, MTT solution (0.5 mg/mL) was added to quantify the number of live and dead cells in each well. The plate was kept at 37°C with 5% CO2 for three hours, during which living cells converted the MTT solution into formazan crystals. The solution in the wells was then removed and the formazan crystals were dissolved in 100 mL of DMSO. The absorbance of the solubilized crystals at 570 nm was measured using a microplate reader made by BioTek in the USA. Based on the following equation, the percentage reduction in cell survival was calculated (16):
The IC50 value (the concentration that inhibits cell growth by 50%) was computed by plotting the concentration against the percentage reduction in cell viability.
3.4. Cellular Fluorescence Staining with Acridine Orange (AO) and Ethidium Bromide (EB)
The cell lines were exposed to the IC50 concentrations of the essential oil for 12 hours. Subsequently, a single-cell suspension was obtained by trypsinization and washing with PBS. The cell suspension was then treated with 100 μL of acridine orange and ethidium bromide solution (1: 100 mg/mL) and visualized under a fluorescence microscope (Zeiss, Germany) (17).
3.5. Analysis of Cellular Apoptosis by Flow Cytometry
For this analysis, cancer cells were cultured with essential oil at its IC50 concentrations. After 48 hours, the cells were trypsinized, rinsed with PBS, and then resuspended in a binding buffer. The cell suspension was then mixed with 5 μL of each fluorescent dye and incubated in a dark environment at room temperature for 15 minutes. Finally, the mixture was analyzed immediately using flow cytometry (Partec PAS, Germany) (18).
3.6. Quantitative Analysis of Data
The reproducibility of the data was confirmed through three independent experiments. Results are expressed as mean and standard deviation for each dataset. Data analysis was performed independently using the SPSS software, employing a t-test (SPSS Inc., Chicago, IL, USA). A P-value of less than 0.05 was considered statistically significant.
4. Results
4.1. GC-MS Analysis of Essential oil Obtained from H. longiflorus
The chromatogram and the compounds identified by GC-MS in the essential oil of H. longiflorus are shown in Appendix 1 (in Supplementary File) and Table 1, respectively. Results revealed that the essential oil contains various compounds with anticancer activity.
| Name of the Compound | Retention Time (min) | Percentage in Essential Oil | Type |
|---|---|---|---|
| α-Thujene | 5.481 | 0.69 | C10H16 (MH) |
| α-Pinene a | 5.611 | 5.17 | C10H16(MH) |
| Sabinene (β-Thujene) | 6.299 | 2.58 | C10H16(MH) |
| L-β-Pinene | 6.352 | 1.47 | C10H16 (MH) |
| Ethylcyclohexane | 6.51 | 2.59 | C8H16 |
| o-Cymene | 7.175 | 0.31 | C10H14 (MH) |
| Carvene(D-Limonene) a | 7.24 | 0.70 | C10H16(MH) |
| 1,8-cineol (eucalyptol) a | 7.281 | 2.70 | C10H18O (OM) |
| Linaloola | 8.393 | 3.13 | C10H18O (OM) |
| Geijerene | 8.957 | 1.60 | C12H18 (SH) |
| L-terpinen-4-ol a | 9.657 | 0.91 | C10H18O (OM) |
| Cyclopentane, 1-hexyl-3-methyl- | 9.763 | 0.99 | C12H24 |
| α –Copaene | 12.481 | 2.42 | C15H24 (SH) |
| β-Bourbonene | 12.622 | 1.84 | C15H24 (SH) |
| Caryophyllene a | 13.092 | 3.80 | C15H24 (SH) |
| β-Copaene | 13.204 | 0.62 | C15H24(SH) |
| Isogermacrene D | 13.398 | 0.42 | C15H24(SH) |
| 1,1,4,8-tetramethyl-cis, cis,4,7,10-cycloundecatriene | 13.528 | 0.34 | C15H24(SH) |
| cis-Muurola-4(14),5-diene | 13.634 | 0.33 | C15H24 (SH) |
| Germacrene D | 13.881 | 5.14 | C15H24 (SH) |
| Bicyclogermacrene | 14.063 | 0.94 | C15H24 (SH) |
| β-bisabolene | 14.133 | 0.69 | C15H24 (SH) |
| γ-Cadinene | 14.269 | 2.57 | C15H24 (SH) |
| 1-Isopropyl-4,7-dimethyl-1,2,3,5,6,8a-hexahydronaphthalene | 14.357 | 2.19 | C15H24(SH) |
| Elemol | 14.751 | 13.43 | C15H26O (OS) |
| Benzoic acid cis-3-hexenyl ester | 14.91 | 0.57 | C13H16O2 |
| Hexadecane (Cetane) | 15.145 | 2.53 | C16H34 |
| Caryophylla-3,8(13)-dien-5α-ol | 15.275 | 0.35 | C15H24O (OS) |
| γ-cadinene | 15.563 | 0.88 | C15H24 (SH) |
| Aromadendrene | 15.651 | 0.91 | C15H24 (SH) |
| Bicyclo[4.4.0]dec-1-ene, 2-Isopropyl-5-methyl-9-methylene | 15.845 | 20.69 | C15H24 (SH) |
| 1H-Indene, 1-ethylideneoctahydro-7a-methyl-, cis- | 16.027 | 5.46 | C9H8 |
| Muurola-4,10(14)-dien-1 β -ol | 16.398 | 1.16 | C15H24O (OS) |
| Biadamantanyliden | 19.951 | 0.30 | C20H28 |
| 3a,9b-Dimethyl-1,2,3a,4,5,9b- hexahydrocyclopenta[a]naphthalen-3-one | 21.809 | 0.43 | C15H18O |
| Namber of Compound | Compound | % | |
| 6 | Monoterpene hydrocarbons (MH) | 10.92 | |
| 16 | Sesquiterpene hydrocarbons (SH) | 45.38 | |
| 3 | Oxygenated monoterpenes (OM) | 6.74 | |
| 3 | Oxygenated sesquiterpenes (OS) | 14.94 | |
| 7 | Others | 12.87 | |
| 35 | Total | 90.85 | |
a The compounds whose cytotoxic effects against cancer cells have been reported.
4.2. Cytotoxicity Evaluation of Essential oil from H. longiflorus on Cancer Cells
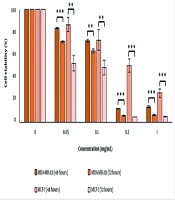
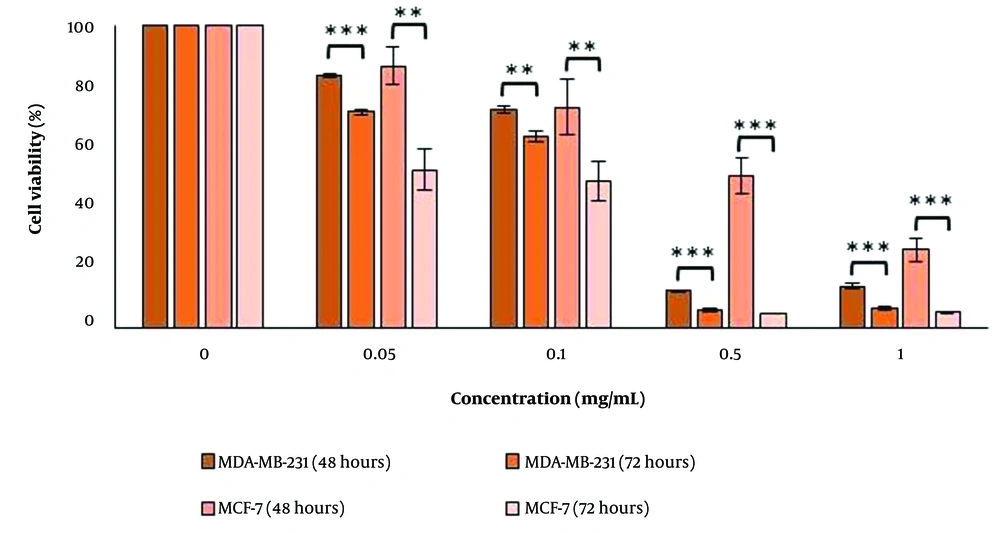
The results demonstrate that treating the cell lines with varying concentrations of the essential oil had both concentration-dependent and time-dependent cytotoxic effects (Figure 1). The IC50 values decreased over time, indicating that the essential oil's effect on the cancer cells is time-dependent (Table 2).
The survival percentage of MDA-MB-231 and MCF-7 cells under varying concentrations of H. longiflorus essential oil in two periods of 48 and 72 hours. The data is based on the mean of three distinct tests. P-value less than 0.05 was considered significant (*P-value < 0.05, **P-value < 0.01, ***P-value < 0.001). Th cells treated with 0.4% Tween were considered negative control.
a IC50 of the essential oil on the MDA-MB-231 cells was compared with their IC50 on the MCF-7 cells in the same period.
b P-value < 0.001 is considered significant.
c P-value < 0.01 is considered significant.
4.3. Evaluation of the Induced Cell Death in MDA-MB-231 and MCF-7 Cell Lines by Essential oil Using Acridine Orange and Ethidium Bromide Double Staining
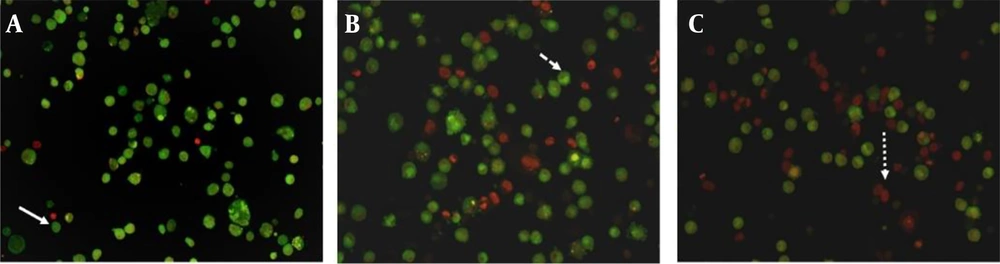
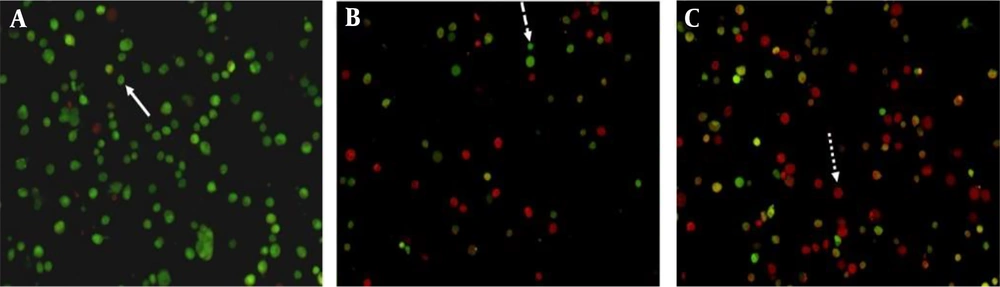
Staining cells with acridine orange and ethidium bromide, and observing them under a fluorescent microscope is a qualitative method used to investigate apoptosis (17). To measure the occurrence of apoptosis, MDA-MB-231 and MCF-7 cells were treated with a negative control (0.4% tween), a positive control (IC50 concentration of carboplatin), and the IC50 concentration of H. longiflorus essential oil. The cells were then stained with acridine orange and ethidium bromide, and images were captured using a fluorescent microscope. As observed, living cells appear green due to the absorption of acridine orange, while dead cells appear red due to the absorption of ethidium bromide (Figures 2 and 3).
Fluorescent microscope image (magnification ×400) of MDA-MB-231 cells treated with A, 0.4% tween; B, carboplatin (positive control) at IC50 concentration and; C, essential oil obtained from H. longiflorus Benth., at IC50 concentration after staining with acridine orange and, ethidium bromide. The smooth arrow indicates live cells, the dashed arrow indicates primary apoptotic cells, and the dotted arrow indicates secondary apoptotic cells.
Fluorescent microscope image (magnification ×400) of MDA-MB-231 cells treated with A, 0.4% tween; B, carboplatin (positive control) at IC50 concentration and; C, essential oil obtained from H. longiflorus, at IC50 concentration after staining with acridine orange and, ethidium bromide. The smooth arrow indicates live cells, the dashed arrow indicates primary apoptotic cells, and the dotted arrow indicates secondary apoptotic cells.
4.4. Quantitatively Investigate the Type of Death of Cells Treated with the Essential Oil
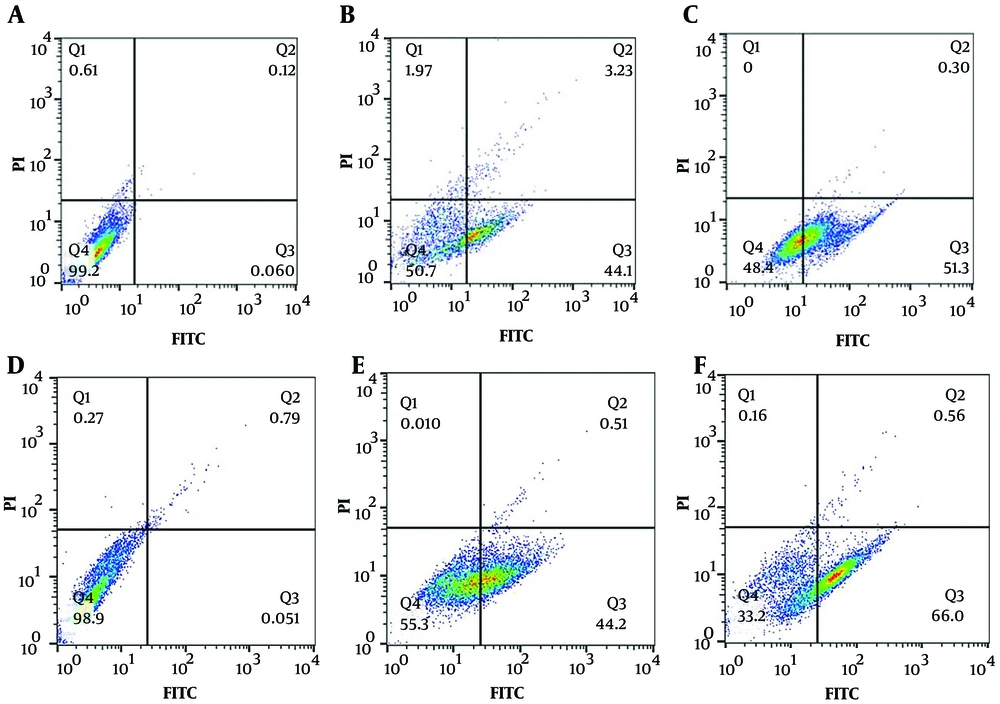
One characteristic of apoptotic cells is a change in the permeability of the cell membrane and the migration of phosphatidylserine from the inner to the outer side of the membrane (18). Annexin V, a protein with a high affinity for phosphatidylserine, is used to detect apoptotic cells when conjugated to FITC, a green fluorescent dye, in flow cytometry. Propidium iodide (PI), a red fluorescent dye that binds to nucleic acids, cannot penetrate the membranes of healthy or apoptotic cells, hence it only stains cells with compromised membranes, such as necrotic cells. To investigate the type of cell death in MDA-MB-231 and MCF-7 cells treated with essential oil from H. longiflorus, the cells were first treated with the IC50 concentration of the essential oil and carboplatin, then stained with Annexin V-FITC and PI, and analyzed using flow cytometry. The results, displayed in Figure 4, show that 51.3% of MDA-MB-231 cells and 66% of MCF-7 cells are in the Q3 area, indicating that the essential oil of H. longiflorus induces apoptosis in MDA-MB-231 and MCF-7 cancer cells.
Flow cytometry analysis of MDA-MB-231 (A, B and C) and MCF-7 (D, E and F) cells treated with 0.4% Tween (negative control) (A and D), carboplatin (positive control) (Band E) and the essential oil (C and F) using two fluorescent dyes, Annexin V-FITC and PI. Q4 (Percentage of live cells ((Annexin V-FITC- and, PI-)), Q3 (Percentage of cells that are in the early stages of apoptosis ((Annexin V-FITC+ and PI-)), Q2 (total percentage of dead cells with apoptosis) delay and, necrosis (Annexin V-FITC+ and, PI+)) and, Q1 (Percentage of necrotic cells (Annexin V-FITC- and, PI+)), FL1-H (related to Annexin V-FITC) and, FL3-H (related to PI).
5. Discussion
Essential oils containing anticancer compounds with minimal side effects are among the most effective agents in cancer treatment (10). Hymenocrater longiflorus is traditionally used to treat certain diseases, and recent studies have reported its antioxidant activity (14). However, no studies have yet explored its anticancer properties in breast cancer. Therefore, this study investigated, for the first time, the cytotoxic properties of H. longiflorus essential oil on two common human breast cancer cell lines: MDA-MB-231 and MCF-7. MDA-MB-231 cells are ER-negative, highly invasive, and metastatic, while MCF-7 cells are ER-positive, highly proliferative, but non-invasive (19). The study revealed that the essential oil of H. longiflorus inhibits the proliferation of both cell types. It had a more significant cytotoxic effect on MDA-MB-231 than on MCF-7 within 48 hours. Over time, the cytotoxic effects on the MCF-7 strain exceeded those on MDA-MB-231, demonstrating that the antiproliferative properties of the essential oil are not dependent on the presence of the estrogen receptor (ER). The inhibitory effect was both concentration and time-dependent.
Double staining of cells with Acridine Orange and Ethidium Bromide is a viability assay that distinguishes dead from viable cells. Acridine Orange penetrates living cells and emits green fluorescence, while Ethidium Bromide stains dead cells and emits orange/red fluorescence (17). Acridine Orange and Ethidium Bromide double staining of MDA-MB-231 and MCF-7 cells treated with the IC50 concentration of the essential oil, compared to the positive and negative controls, confirmed cell death induction by the essential oil in both cell lines. A quantitative and more accurate evaluation of the type of cell death was performed using flow cytometry after staining the cells with Annexin V-FITC and PI. As seen in the flow cytometry results (Figures 4), in the negative control sample, more than 90% of the cells lacked fluorescent dye in the Q4 region. In contrast, in cells treated with carboplatin (positive control) and the essential oil, between 40 and 66% of the cells were in the Q3 region, indicating the movement of phosphatidylserine from the inner part of the cell membrane to the outer part as a result of apoptosis, where it attached to the Annexin V-FITC dye. Therefore, the essential oil of H. longiflorus has caused the death of MDA-MB-231 and MCF-7 cancer cells by inducing apoptosis.
Studies on the anticancer properties of Hymenocrater species are limited. The quantity and quality of secondary metabolites in the genus Hymenocrater depend on factors such as genetic structure, location, growing conditions, harvest season, and extraction methods (20, 21). Ahmadi et al. identified phenolic, terpenic, and antioxidant compounds in H. longiflorus Benth. essential oil from the Oraman mountains in Kermanshah, noting a direct relationship between the phenolic content of the essential oil and its antioxidant properties. Phenolic compounds are crucial biologically active components of the plant that can inhibit lipid oxidation at an early stage. H. longiflorus Benth. essential oil also contains antibacterial compounds like 1,8-cineole (eucalyptol) and antifungal compounds such as oxygenated monoterpenes (14). In various studies, the essential oil of H. longiflorus from Kurdistan and Kermanshah provinces has been analyzed by GC-MS, revealing that the composition of essential oils can vary due to factors like geographical location, altitude, soil type, and the timing and methods of plant harvesting and essential oil extraction (13). Extensive research has explored the impact of compounds found in essential oils on various cancer cell lines, showing promising anti-cancer properties, including anti-angiogenesis, anti-metastasis, and the inhibition of tumor proliferation and growth. Specifically, α-pinene, a monoterpene derived from the essential oil of pine needles, has been found to decrease the expression of MMP-9 in the three-dimensional spheroids of MDA-MB-231 breast cancer cells through the down-regulation of NF-κB, thus inhibiting the invasion of metastatic MDA-MB-231 cells (22).
5.1. Conclusions
The results of the present study revealed that the essential oil of H. longiflorus Benth. contains various anticancer and medicinal compounds capable of inducing apoptosis in MDA-MB-231 and MCF-7 human breast cancer cell lines.