1. Background
Breast cancer (BC) is the most commonly diagnosed malignancy globally, with an estimated 2.3 million new cases reported in 2020, surpassing lung cancer for the first time (1). The incidence and mortality rates of cancer are increasing rapidly worldwide, primarily due to increased life expectancy, population growth, and socioeconomic development (2, 3). In Iran, BC is the most prevalent type of cancer among women, with an incidence rate of 9 per 100,000 people (4). Survival rates for BC vary significantly, with low-income countries accounting for over 50% of BC mortality due to limited resources and treatment infrastructure (5, 6).
Early diagnosis and understanding the genetic and environmental risk factors are crucial for effective prevention and management of BC (7). Genetic factors, including inherited mutations and common genetic variations (polymorphisms), significantly contribute to BC susceptibility (8, 9). The BRCA2 gene, located at the 13q13.1 locus, is a well-known tumor suppressor gene associated with hereditary breast and ovarian cancer (10). The BRCA2 protein is involved in various cellular processes, including transcription, cell cycle regulation, DNA repair, mitophagy, and replication fork stabilization (11).
While extensive research has been conducted on the association between BRCA2 gene mutations and BC risk, few studies have investigated the role of BRCA2 polymorphisms in the Iranian population (12, 13). The ClinVar database currently lists 4,956 pathogenic variants in the BRCA2 gene (14). This study aims to evaluate the association between two specific BRCA2 polymorphisms, rs11571833 (c.9976A>T, p.Lys3326Ter) and rs4987117 (c.865A>C, p.Asn289His), and the risk of BC in the Iranian population. These variants were selected due to their potential functional significance and reported associations with BC in other populations (15, 16). The rs11571833 variant is a nonsense mutation resulting in a premature stop codon, while the rs4987117 variant is a missense mutation with a minor allele frequency (MAF) of 0.05 in the Asian population (17, 18).
2. Objectives
The findings of this study will contribute to a better understanding of the genetic factors influencing BC risk in the Iranian population and may have implications for early detection, risk assessment, and personalized prevention strategies.
3. Methods
3.1. Ethics
The ethics committee of the Islamic Azad University of Neyshabur approved the study protocol with the ethical approval number IR.IAU.NEYSHABUR.REC.1401.012. Each participant signed a consent form before commencing the study and had the right to withdraw at any time. The checklists were collected in a manner that did not reveal the identities of the research participants, and all data obtained from the research community were kept confidential.
3.2. Sample Selection
In this study, 50 BC patients from Omid Hospital and 50 healthy controls from Shariati Hospital’s screening program in Mashhad were selected. Patients were included if they had a confirmed BC diagnosis, no genetic disorders related to BC, and were not undergoing active treatment. Exclusion criteria for patients included previous BC recurrence, metastatic disease at diagnosis, or participation in another clinical trial. Controls were included if they had no personal or first-degree family history of cancer and were aged 30 - 60 years, matching the patients’ age range. Controls were excluded if they had significant medical conditions affecting genetic analysis, a history of genetic disorders, or had undergone hormone replacement therapy within the last six months. A specialist confirmed the health of the controls, and demographic and clinical data were collected via questionnaires for all participants.
3.3. Blood and DNA Preparation
The DNA was extracted from 5 mL of peripheral blood using the DNA extraction kit (Parstous Co.). The quality of the extracted DNA was analyzed by loading it onto a 1% agarose gel, and its concentration was measured using an Epoch™ microplate spectrophotometer.
3.4. Genotyping
The amplification refractory mutation system (ARMS) method was used to genotype rs11571833 and rs4987117. The primer sequences used in this study were referenced from Ghadirkhomi et al. (17). The electrophoresis of the PCR products was carried out in a 2% agarose gel. The PCR reaction was performed in a 20 μL reaction volume containing 6 μL master mix, 1 μL of each primer (10 μM), 5.5 μL deionized water, and 5.5 μL (100 ng) genomic DNA. PCR conditions for both wild and mutant types of rs11571833 included an initial cycle at 95°C for 10 min, followed by 30 cycles at 95°C for 1 min, 56.5°C for 45 s, and 72°C for 40 s, and a final extension at 72°C for 5 min. For rs4987117, the conditions included an initial cycle at 95°C for 3 min, followed by 30 cycles at 95°C for 30 s, 47.5°C for 40 s, and 72°C for 1 min, with a final extension at 72°C for 5 min.
3.5. Statistical Analysis
The deviation of allelic frequencies from the Hardy-Weinberg equilibrium was checked using Pearson's χ2 distribution with one degree of freedom. Various genetic analysis models were utilized to evaluate the associations of BC risk, demographic data, and tumor characteristics with genotypes through binary logistic regression. Multivariate logistic regression was employed to assess the association of independent variables with cancer risk, reported as odds ratios (ORs) with 95% confidence intervals. Other statistical analyses were conducted using SPSS 16.0 (IBM, USA). P-values less than 0.05 were considered significant.
4. Results
4.1. Demographic and Clinical Characteristics
Table 1 presents the demographic characteristics of the case and control groups. The mean age of BC patients was 49.38 ± 9.98 years, significantly lower than the mean age of controls (53.84 ± 9.41 years, P = 0.027). Significant differences were also observed in physical activity levels (P = 0.041) and Body Mass Index (BMI) (P = 0.004), with BC subjects exhibiting lower levels of physical activity and higher BMI compared to healthy controls. Table 2 summarizes the clinical characteristics of the BC patients. Cytopathological evaluation revealed that 72% of patients were diagnosed with early-stage disease (stages I & II), and 56% had grade II tumors. The most frequent tumor subtype was invasive ductal carcinoma (75.2%), followed by invasive lobular carcinoma (5.8%).
| Characteristics | Control Group | Case Group | P-Value | OR (95%CI) |
|---|---|---|---|---|
| Age | 53.84 ± 9.41 | 49.38 ± 9.98 | 0.027 | 1.05 (1.01 - 1.09) |
| Menarche age | 13.42 ± 1.82 | 13.16 ± 1.56 | 0.441 | 1.10 (0.87 - 1.39) |
| Body Mass Index (BMI) | 26.73 ± 4.62 | 29.64 ± 4.63 | 0.004 | 0.87 (0.79 - 0.96) |
| History of iactation | 2.00 (0.35 - 11.47) | |||
| Positive | 45 (90.0) | 45 (90.0) | Reference | |
| Negative | 2 (4.0) | 4 (8.0) | 0.437 | |
| Status of activity | 0.37 (0.14 - 0.96) | |||
| Positive | 42 (84.0) | 33 (66.0) | Reference | |
| Negative | 8 (16.0) | 17 (34.0) | 0.041 | |
| Status of occupation | 0.53 (0.21 - 1.32) | |||
| Housewife | 34 (68.0) | 40 (80.0) | Reference | |
| Employee | 16 (32.0) | 10 (20.0) | 0.174 | |
| Education | 0.58 (0.24 - 1.38) | |||
| Academic | 18 (36.0) | 12 (24.0) | Reference | |
| Non-academic | 32 (64.0) | 37 (74.0) | 0.215 |
Demographic Features in Breast Cancer and Healthy Groups
| Characteristics | No. (%) |
|---|---|
| Tumor type | |
| Invasive ductal carcinoma | 38 (75.2) |
| Ductal carcinoma in situ | 4 (6.5) |
| Invasive lobular carcinoma | 3 (5.8) |
| Grade | |
| I | 12 (24) |
| II | 28 (56) |
| III | 10 (20) |
| Stage | |
| I | 11 (22) |
| II | 25 (50) |
| III | 11 (22) |
| IV | 3 (6) |
Frequency Distribution of Tumor Characteristics
4.2. Association of BRCA2 Gene Variants with Breast Cancer Risk
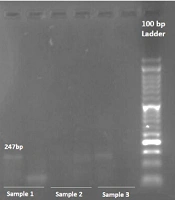
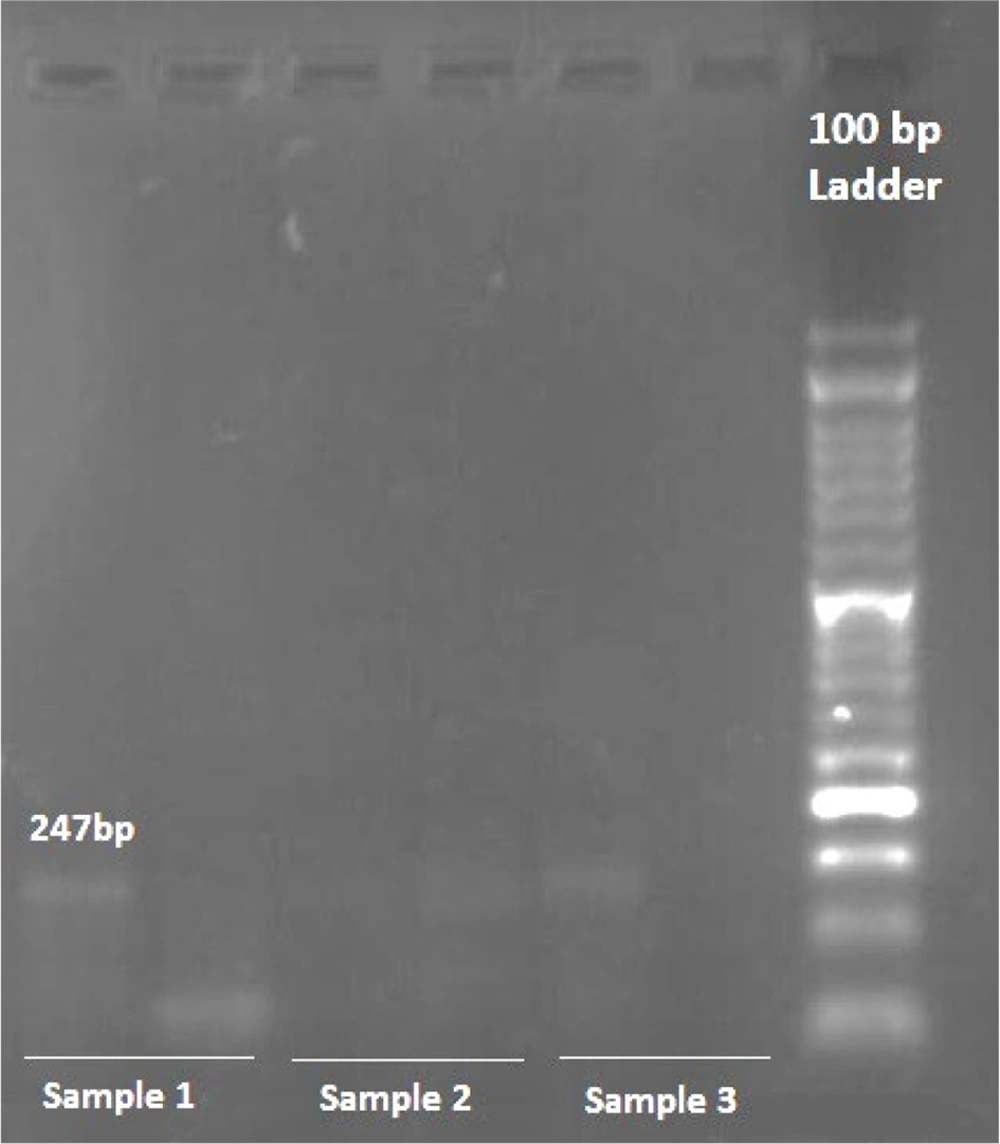
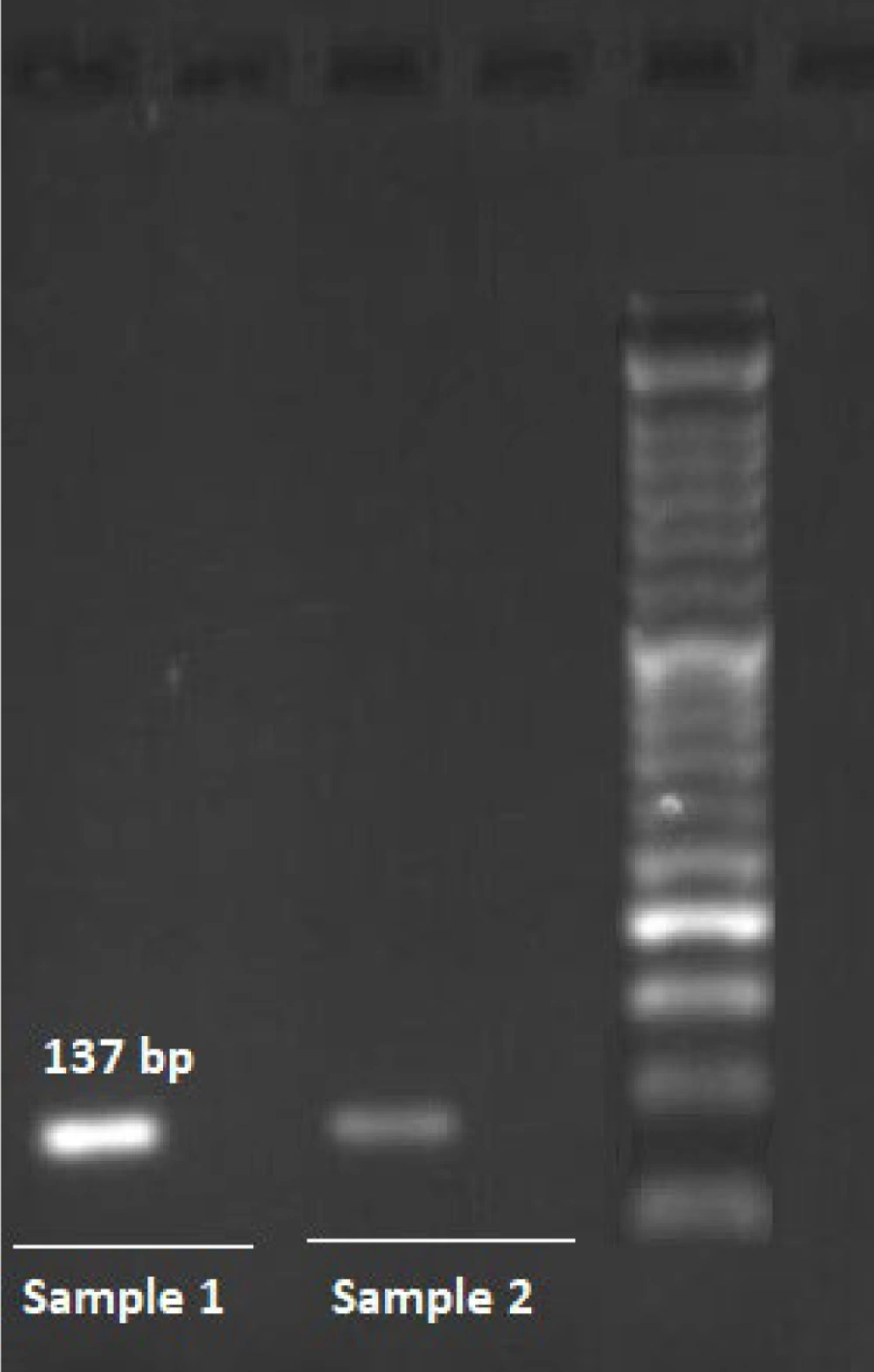
This study investigated the association of two single nucleotide polymorphisms (SNPs), rs11571833 and rs4987117, with BC risk, utilizing allele-specific PCR (AS-PCR) for genotyping. Representative photomicrographs of the AS-PCR results for rs11571833 and rs4987117 are presented in Figures 1, and 2, respectively. Analysis of rs11571833 revealed a statistically significant association with BC risk. The AT genotype exhibited a reduced odds ratio (OR) of 0.36 (95% CI: 0.14 - 0.92, P = 0.003), the TT genotype an OR of 0.19 (95% CI: 0.04 - 0.98, P = 0.047), and the allelic model (A vs. T) an OR of 2.89 (95% CI: 1.43 - 5.84, P = 0.003), suggesting a protective effect of the AT and TT genotypes and the T allele. The TT genotype was significantly more frequent in BC patients (14%) compared to controls (4%), while the AT genotype was observed in 36% of cases and 20% of controls. The AA genotype was more prevalent in the control group (76%), and the recessive model (AA vs. AT + TT) showed a significant association with BC risk, with an OR of 0.32 (95% CI: 0.13 - 0.74, P = 0.008), indicating a protective effect of the AA genotype (Table 3 and 4). In contrast, the rs4987117 polymorphism did not demonstrate a significant association with BC risk in any of the genetic models tested (P > 0.05) (Table 5 and 6). These findings suggest a potential role for rs11571833 in BC risk, warranting further investigation to elucidate its functional implications and validate these results in larger, independent cohorts.
| Genotype | Case (n = 50) | Control (n = 50) | P-Value | OR (95% CI) |
|---|---|---|---|---|
| AA | 25 (50) | 38 (76) | Reference | - |
| AT | 18 (36) | 10 (20) | 0.033 | 0.36 (0.14 - 0.92) |
| TT | 7 (14) | 2 (4) | 0.047 | 0.19 (0.04 - 0.98) |
| Allele | Frequency | Frequency | P-value | OR (95% CI) |
| T | 32 (32) | 14 (14) | Reference | - |
| A | 68 (68) | 86 (86) | 0.003 | 2.89 (1.43 - 5.84) |
Genotype and Allele Frequencies of rs11571833 in Cases and Controls a
| Genetic Model | Case (n = 50) | Control (n = 50) | P-Value | OR (95% CI) |
|---|---|---|---|---|
| Dominant | ||||
| AA + AT | 43 (86) | 48 (96) | Reference | - |
| TT | 7 (14) | 2 (4) | 0.100 | 3.91 (0.77-19.83) |
| Recessive | ||||
| AT + TT | 25 (50) | 12 (24) | Reference | - |
| AA | 25 (50) | 38 (76) | 0.008 | 0.32 (0.13 - 0.74) |
Association of rs11571833 Genotypes with Breast Cancer Risk in Different Genetic Models a
| Genotype | Case (n = 50) | Control (n = 50) | P-Value | OR (95% CI) |
|---|---|---|---|---|
| CC | 23 (46) | 31 (62) | Reference | - |
| CT | 21 (42) | 17 (34) | 0.232 | 0.60 (0.26 - 1.39) |
| TT | 6 (12) | 2 (4) | 0.105 | 0.25 (0.05 - 1.34) |
| Allele | Frequency | Frequency | P-value | OR (95% CI) |
| T | 33 (33) | 21 (21) | Reference | - |
| C | 67 (67) | 79 (79) | 0.058 | 1.58 (0.98 - 3.50) |
Genotype and Allele Frequencies of rs4987117 in Cases and Controls a
| Genetic Model | Case (n = 50) | Control (n = 50) | P-Value | OR (95% CI) |
|---|---|---|---|---|
| Dominant | ||||
| CC + CT | 44 (88) | 48 (96) | Reference | - |
| TT | 6 (12) | 2 (4) | 0.159 | 3.27 (0.63 - 17.07) |
| Recessive | ||||
| CT + TT | 27 (54) | 19 (38) | Reference | - |
| CC | 23 (46) | 31 (62) | 0.110 | 0.52 (0.23 - 1.16) |
Association of rs4987117 Genotypes with Breast Cancer Risk in Different Genetic Models a
5. Discussion
This study investigated the association of two BRCA2 gene variants, rs11571833 and rs4987117, with BC risk in the Iranian population. Our findings revealed a significant association between the rs11571833 variant and increased BC risk, particularly for the AT and TT genotypes. The association was stronger for estrogen receptor-negative BC and serous ovarian cancer, suggesting a potential role of this variant in modifying BC subtypes. This observation aligns with previous studies, including those by Thompson et al. (19) and Meeks et al. (as cited in Arbustini)(20), which demonstrated a link between this variant and BC susceptibility, particularly in BRCA1 mutation carriers.
However, no significant association was found between the rs4987117 variant and BC risk in this study, consistent with a meta-analysis by Faramarzi et al. (21). This finding contradicts some previous studies, such as those by Kovacheva et al. (22) and Solodskikh et al. (23), which reported an association between this variant and BC risk. However, these studies differed in their populations and methodologies, potentially contributing to the conflicting results. Furthermore, Johnson et al. (24) did not identify rs4987117 among 21 variants associated with BC in their analysis of 1037 functional SNPs. These discrepancies underscore the complex interplay between genetic factors and BC risk, highlighting the need for further research to clarify the role of rs4987117 in different populations.
The present study also supports previous findings regarding the influence of age, BMI, and physical activity on BC risk. The significantly lower mean age of BC patients in this study, aligning with other Iranian studies (25, 26), suggests an earlier onset of BC in this population compared to neighboring countries. The observed association between increased BMI and BC risk aligns with global studies (27), suggesting a shared risk factor across diverse populations. Similarly, the lower physical activity levels among BC patients are consistent with findings demonstrating the protective effect of regular exercise against BC (28). The observed differences in the frequency of polymorphisms between the Iranian population and other populations, as highlighted in the results, indicate potential variations in population genetics and the impact of alleles on specific traits. Further research is needed to elucidate the specific genetic and environmental factors contributing to BC risk in the Iranian population and to develop tailored prevention and early detection strategies.
This study provides valuable insights into the association between specific BRCA2 gene variants and BC risk in the Iranian population. The significant association observed for rs11571833 highlights the potential utility of this variant for BC risk assessment in this population. However, the lack of association for rs4987117 suggests that further investigation is needed to clarify its role in BC development in different populations. The findings also underscore the importance of considering demographic and lifestyle factors, such as age, BMI, and physical activity, in understanding BC risk and developing effective prevention strategies.
5.1. Conclusions
This study investigated the association of two BRCA2 gene variants, rs11571833 and rs4987117, with BC risk in the Iranian population. Our findings suggest that the rs11571833 variant may be associated with an increased risk of BC, particularly for the AT and TT genotypes. This observation aligns with previous studies highlighting the potential role of this variant in BC susceptibility. However, no significant association was found for the rs4987117 variant. While these findings warrant further investigation, they underscore the potential importance of BRCA2 polymorphisms in BC susceptibility within the Iranian population. Understanding the functional impact of these variants could contribute to personalized risk assessment and potentially lead to the identification of novel therapeutic or diagnostic targets. Further research with larger sample sizes and comprehensive analyses of additional BRCA2 variants is crucial to confirm these findings and elucidate the complex interplay between genetic factors and BC risk in this population.