1. Background
Obesity is one of the most prevalent issues in modern society, primarily caused by reduced physical activity and unhealthy dietary habits (1). Weight gain and fat accumulation in obesity are often accompanied by a loss of muscle mass, which alters body composition and further increases fat mass (2). These changes raise the risk of developing obesity-related conditions such as cardiovascular disease, type 2 diabetes, and insulin resistance (3). The reduction in muscle mass due to obesity can negatively affect metabolic and physiological systems (4). Skeletal muscle secretes soluble factors known as myokines, which play a role in regulating metabolic pathways and physiological processes in other tissues (5).
Myonectin, also referred to as C1Q/TNF-related protein 15 (CTRP15), is a well-known myokine that is sensitive to nutrient levels (6). Studies have shown that myonectin not only regulates lipid metabolism but also plays a significant role in the erythropoietic stress response, particularly in iron mobilization within the liver (7). Additionally, myonectin inhibits macrophage inflammation and cardiac apoptosis, contributing to the prevention of acute myocardial ischemic injury (6, 7). Its secretion from skeletal muscles enhances free fatty acid consumption, metabolism, and overall quality of life, while reducing cardiovascular risk factors (8).
Physical activity and nutrition influence myonectin expression, which promotes the uptake of plasma free fatty acids into adipose tissue through the expression of fatty acid transporters (FATP412, FATP) (8). Myonectin regulates metabolic interactions between adipose and muscle tissues (9), with levels decreasing during fasting and rising in response to glucose or lipid intake (10). Furthermore, myonectin is involved in the phosphorylation of AMPK2, stimulation of fatty acid oxidation, glucose uptake via glucose transporters, and overall regulation of energy balance, glucose and fat metabolism, and insulin sensitivity (6). Alterations in circulating myonectin levels can therefore affect a wide range of physiological processes. Myonectin is an essential regulator of the glucose metabolism (11). Disturbances in myonectin secretion have been linked to the development of insulin resistance (12). Regular physical activity plays a protective role against chronic metabolic diseases such as type 2 diabetes and insulin resistance (13). Numerous studies have demonstrated that both high-intensity and moderate-intensity exercise stimulate the secretion of various factors from tissues across different body regions, with secretion levels varying according to exercise intensity (6, 14). For instance, previous research has shown that high-intensity interval training (HIIT) exerts a greater influence on tissue signaling modulation compared to resistance training (15). Myonectin levels tend to decrease in individuals with low levels of physical activity, whereas physical activity increases myonectin levels (16). Another study found that myonectin stimulates glucose transporters in skeletal muscle and increases adenosine monophosphate kinase 3 activity, thereby enhancing glucose uptake (17).
In contrast, voluntary wheel running for two weeks was shown to increase myonectin levels in serum and its expression in the gastrocnemius and soleus muscles of rats (18). Additionally, new findings indicate that myonectin gene expression decreased after nine weeks of endurance training (19). However, a separate study documented a rise in myonectin gene expression following a single session of exercise (20). Myonectin levels have been reported to be lower in patients with type 2 diabetes and obesity (21, 22), but physical activity has been shown to elevate both the expression and circulation of myonectin in skeletal muscle (22). Despite these findings, limited research has compared the effects of HIIT and moderate-intensity continuous training (MICT) on myonectin-dependent responses.
2. Objectives
Given the role of muscle contraction in myokine synthesis and secretion, this study aimed to compare the effects of continuous moderate-intensity exercise and high-intensity interval training (HIIT) on myonectin levels in desert rats.
3. Methods
3.1. Animal Care
For the present study, 24 male desert rats with an average age of 18 to 20 weeks and an average weight of 332 ± 353 grams were purchased from the Pasteur Institute in Tehran (23). The animals were randomly divided into three groups—control, continuous exercise, and high-intensity interval exercise-based on weight matching, with 8 rats in each group (23). These animals were housed in cages, with 4 rats per cage, in an environment maintained at an average temperature of 22 ± 2 degrees Celsius, with approximately 60% humidity and a 12-hour light-dark cycle. Throughout the study period, the animals had free access to both water and food (23).
3.2. Training Protocol
After one week of familiarization with the laboratory environment and treadmill exercise (10 minutes of running at a speed of 10 meters per minute), the exercise groups followed their respective exercise protocols for 8 weeks (24). The continuous exercise group began their program with 12 minutes of running during the first session, gradually increasing to approximately 15 minutes with a 5-degree incline in subsequent sessions (25). The incline and running duration were adjusted to be equivalent to those of the high-intensity interval exercise group (24, 25). From the start of the sixth week until the end of the protocol, the exercise intensity and volume remained constant. During this period, the animals in the continuous exercise group performed 54 minutes of treadmill activity at a speed of 20 meters per minute with a 15-degree incline (26).
The high-intensity interval exercise program lasted 6 weeks and involved progressive changes in incline, treadmill speed, and the number of intervals, reaching 6 intervals of 3 minutes each at a speed of 40 meters per minute with 3 minutes of active rest at a speed of 20 meters per minute and a 15-degree incline by the beginning of the sixth week (26). The protocol remained consistent from the sixth week until the end of the program (26). The first session for this group consisted of 2 intervals of exercise at a speed of 15 meters per minute with active rest at 12 meters per minute and a 5-degree incline. Speed was gradually increased by 1 to 2 meters per minute in each session (27). Additionally, one interval was added each week, reaching 6 intervals by the fifth week (27). The treadmill incline increased by 5 degrees each week until the third week. Each exercise session for both groups began with a 5-minute warm-up at a speed of 10 meters per minute and concluded with the same program for a cool-down (27).
3.3. Sample Collection
Blood sampling and tissue collection were conducted 72 hours after the final exercise session. The animals were anesthetized via intraperitoneal injection of a combination of ketamine (50 mg per kilogram of body weight) and xylazine (3 - 5 mg per kilogram of body weight). Approximately 6 milliliters of blood were drawn from the jugular vein of each animal, and after allowing the blood to clot, it was centrifuged at 3000 rpm for 15 minutes to separate the serum. The separated serum samples were stored in a freezer at -20°C for subsequent analysis. In addition, the gastrocnemius and long finger flexor muscles, as well as the epididymal and retroperitoneal fat tissues, were isolated and weighed. Serum glucose levels were measured using an enzymatic-colorimetric method (Pars Azmoon Company) (23).
3.4. Blood and Tissue Measurements
The measurement of serum insulin and myonectin levels was performed using an ELISA method (Rat Myonectin (CTRP15) ELISA Kit, EASTBIOPHARM. Sensitivity: 0.026 ng/ml, Intra-Assay CV < 10%, Inter-Assay CV < 12%) from Istituto BioPharma. Similarly, the serum insulin levels were measured using the Rat Insulin (INS) ELISA Kit (EASTBIOPHARM. Sensitivity: 0.05 mIU/L, Intra-Assay CV < 10%, Inter-Assay CV < 12%). The Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) index was calculated using the following formula (23):
HOMA-IR = (fasting serum insulin (μU/mL) × fasting plasma glucose (mmoll-1))/22.5
The weights of the Flexor hallucis longus (FHL) muscle, gastrocnemius muscle, as well as the epididymal and retroperitoneal adipose tissues, were measured after removal.
3.5. Statistical Analysis
Statistical analysis of the research data was first conducted to ensure normal distribution using the Kolmogorov-Smirnov test. One-way analysis of variance (ANOVA) followed by the LSD post-hoc test was applied to examine differences between groups. The Pearson correlation test was used to assess the correlation between serum myonectin levels, insulin resistance index, and other evaluated variables. All statistical analyses were performed using SPSS version 16, with a significance level of P ≤ 0.05 considered for all tests (23).
4. Results
Information regarding body weight changes in the different research groups, along with relative muscle (gastrocnemius and FHL) and fat tissue (epididymal and retroperitoneal) weights, is presented in Table 1. The significant weight gain observed during the research protocol was notably lower in both the continuous exercise group (P = 0.010) and the high-intensity interval exercise group (P = 0.009) compared to the control group. There was no significant difference in relative gastrocnemius muscle weight between the groups, but the relative FHL muscle weight was higher in the high-intensity interval exercise group compared to the control group (P = 0.007). The relative weight of epididymal fat tissue was significantly lower in the continuous exercise and high-intensity interval exercise groups compared to the control group (P = 0.007 and P = 0.001, respectively). Similarly, the relative weight of retroperitoneal fat tissue was significantly reduced in both the continuous exercise and high-intensity interval exercise groups compared to the control group (P = 0.001 and P < 0.001, respectively). No significant difference in relative tissue weights was found between the two exercise groups.
| Variables | Control | MICT | HIIT |
|---|---|---|---|
| Weight change (gr) | 59.7 ± 20.3 | 34.5 ± 18.8 b | 34.1 ± 13.4 b |
| relative weight of gastrocnemius muscle (%) | 1.22 ± 0.08 | 1.22 ± 0.04 | 1.25 ± 0.07 |
| Relative weight of FHL muscle (%) | 0.29 ± 0.02 | 0.30 ± 0.01 | 0.31 ± 0.02 b |
| Relative weight of epididymal fat (%) | 1.43 ± 0.01 | 1.17 ± 0.02 b | 1.08 ± 0.01 b |
| Relative weight of retroperitoneal fat (%) | 1.23 ± 0.02 | 0.87 ± 0.02 b | 0.80 ± 0.01 b |
Abbreviations: MICT, moderate-intensity continuous training; HIIT, high-intensity interval training.
a Values are expressed as mean ± SD.
b Statistical difference in comparison with the control group (P < 0.05); and statistical difference compared to the continuous training group (P < 0.05).
No significant differences in fasting serum glucose levels were observed between the groups (Table 2). However, serum insulin levels and the insulin resistance index were significantly reduced in the high-intensity interval exercise group compared to the control group (P < 0.001 and P = 0.005, respectively). These levels were also significantly lower in the high-intensity interval exercise group compared to the continuous exercise group (P < 0.001 and P = 0.029, respectively). Although the mean serum myonectin levels were higher in the exercise groups compared to the control group, these differences were not statistically significant (P > 0.05).
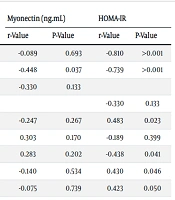
The Pearson correlation test was used to assess the correlation between serum myonectin levels, the insulin resistance index, and other variables (Table 3). A significant inverse correlation between serum myonectin levels and insulin was found (P = 0.037, r = -0.448). Additionally, a significant inverse correlation was observed between the insulin resistance index and serum glucose levels, insulin levels, and relative FHL muscle weight (P < 0.05). Changes in body weight and relative fat tissue weights also showed a direct and significant correlation with the insulin resistance index (P ≤ 0.05).
Abbreviations: MICT, moderate-intensity continuous training; HIIT, high-intensity interval training.
a Values are expressed as mean ± SD.
b Statistical difference in comparison with the control group (P < 0.05); and statistical difference compared to the continuous training group (P < 0.05).
| Variables | Myonectin (ng.mL) | HOMA-IR | ||
|---|---|---|---|---|
| r-Value | P-Value | r-Value | P-Value | |
| Fasting blood glucose (mg/dL) | -0.089 | 0.693 | -0.810 | > 0.001 |
| Insulin (microunits/mL) | -o.448 | 0.037 | -0.739 | > 0.001 |
| HOMA-IR | -0.330 | 0.133 | ||
| Myonectin (ng/mL) | -0.330 | 0.133 | ||
| Weight change (gr) | -0.247 | 0.267 | 0.483 | 0.023 |
| Relative weight of gastrocnemius muscle (%) | 0.303 | 0.170 | -0.189 | 0.399 |
| Relative weight of FHL muscle (%) | 0.283 | 0.202 | -0.438 | 0.041 |
| Relative weight of epididymal fat (%) | -0.140 | 0.534 | 0.430 | 0.046 |
| Relative weight of retroperitoneal fat (%) | -0.075 | 0.739 | 0.423 | 0.050 |
5. Discussion
The results of the present study showed that serum myonectin levels increased in the exercise groups compared to the control group, suggesting a possible link to an excessive increase in leptin; this may be due to the chronic stimulation of muscle cells by leptin (10). Leptin has recently been shown to stimulate the myonectin gene in muscle cells (10), although leptin levels were not measured in this study. Additionally, serum myonectin levels were higher in the high-intensity interval exercise rats compared to the moderate-intensity continuous exercise rats. According to this study, eight weeks of resistance training and HIIT at moderate intensity, regardless of training volume, significantly elevated myonectin levels in the exercise groups compared to the control group.
In a separate 2012 study, Seldin et al. reported increased myonectin expression and systemic circulation in male C57BL/6 rodents after two weeks of exercise, which is consistent with the findings of the current study (6). However, a different study using the Zucker model showed opposing results. This research, which involved nine weeks of aerobic exercise on a treadmill, concluded that myonectin gene expression significantly decreased in both lean and obese rodents, regardless of obesity status (19). A notable factor that may explain these discrepancies is the variation in exercise duration and diet (calorie-restricted vs. high-fat diets) used in the studies. In contrast, our study found that serum myonectin levels increased in all rodents after an eight-week exercise intervention (moderate-intensity resistance combined with high-intensity intervals).
The differences in myonectin levels observed in prior studies may be attributed to disparities in animal models, variations in leptin function, or differences in exercise modalities (19, 26, 28, 29). Overall, the findings suggest that leptin may play a role in regulating myonectin expression, with nutritional status potentially stimulating its secretion. Several studies have linked insulin resistance in skeletal muscle to alterations in the expression and secretion of myokines (30). These changes could disrupt lipid and glucose metabolism in adipose tissue, leading to an abnormal cycle of myokine synthesis and insulin resistance (28, 30). While muscles directly enhance GLUT4 transport and glycogen synthesis in response to insulin, myokines also influence glucose and lipid metabolism throughout the body and contribute to energy balance (7, 30). Additionally, they act on fat tissue, the liver, stomach, and intestines (18).
As demonstrated in this study, the observed reduction in insulin resistance and the increase in myonectin and insulin levels following high-intensity interval exercise (which were not statistically significant in the moderate-intensity resistance group) align with previous research findings. Variations in circulating myonectin levels may influence a broad range of physiological processes (4, 10).
In the present study, exercise interventions increased serum myonectin levels. We also found that this myokine returned to normal levels after eight weeks of both HIIT and moderate-intensity resistance training. Moreover, an inverse correlation was identified between myonectin concentration and glucose levels, HOMA-IR score, and adipose weight (30). On the other hand, a positive correlation was observed between myonectin concentration and the weight of the FHL and gastrocnemius muscles. Few studies to date have investigated the relationship between exercise and myonectin levels (6). Animal studies have shown that voluntary wheel running for two weeks and endurance treadmill training for four weeks increased myonectin levels in skeletal muscle and blood circulation in wild-type mice (29). Additionally, after nine weeks of aerobic exercise, an increase in myonectin concentration in the diaphragm muscle of lean and obese Zucker mice was observed (29). Furthermore, eight weeks of aerobic exercise increased serum myonectin levels in obese women, as reported in a human study. Prior research has primarily focused on low to moderate aerobic exercise. In this study, we explored the effects of moderate-intensity continuous exercise and high-intensity interval exercise.
5.1. Conclusions
The results of this study demonstrate that eight weeks of exercise-regardless of volume or type-can increase myonectin levels and reduce insulin resistance across all exercise groups. Therefore, even moderate-intensity continuous exercise appears to produce similar improvements in insulin resistance and serum myonectin levels. Further research is necessary to expand upon these findings.