1. Background
About one-third of the world's population is currently obese or overweight (1). Excessive calorie consumption is the primary cause of obesity (2). Several studies have shown that rats fed a high-fat diet (HFD) with fructose exhibit dyslipidemia, visceral fat accumulation, impaired glucose tolerance, and ventricular hypertrophy (3, 4). Previous research confirms that an HFD contributes to cardiovascular disease (5).
Obesity causes inflammation and cardiovascular disease through various pathways. Therefore, targeting these pathways can protect cardiomyocytes and enhance cardiac repair mechanisms (6, 7). Nuclear factor Kappa B (NF-κB) regulates the expression of inflammatory cytokines and anti-inflammatory genes during the resolution of inflammation in vivo. The chronic progression of hypertrophy, fibrosis, and ventricular dysfunction continues with a local increase in cytokines and NF-κB activation (8). Blocking NF-κB reduces heart failure and promotes the regeneration of myocardial infarction (9). The enzyme Cyclooxygenase-2 (COX-2) is targeted with NF-κB and regulates prostaglandin generation from arachidonic acid (10). Cyclooxygenase-2 is rapidly induced as part of inflammatory reactions in response to extracellular stimuli and regulates cell proliferation, differentiation, and carcinogenesis (11). Inhibition of COX-2 reduces cardiovascular and inflammatory complications in obese mice treated with monosodium glutamate (12). Nuclear factor Kappa B and COX-2 are the main pathways for chronic inflammation, and the targeted inhibition of NF-κB and COX-2 is considered a practical goal in treating inflammatory diseases (13).
Regular exercise regulates inflammatory signaling cascades and is associated with anti-inflammatory markers (14). Long-term training reduces inflammatory cytokines and mild chronic inflammation by decreasing the levels of NF-κB and COX-2 (15).
Despite the obvious benefits of regular exercise in controlling obesity and preventing cardiovascular diseases, there are conflicting results regarding the impact of regular aerobic activity on COX-2 and NF-κB genes, which are involved in inflammatory pathways (15-19). These contradictory results may be due to differences in the duration of the training period or the intensity of the exercises used. In addition, limited information is available on the impact of increasing aerobic exercise (IAE) performed by gradually increasing the load on inflammatory factors in heart tissue.
2. Objectives
Therefore, this study seeks to determine whether IAE affects the mRNA expression of COX-2 and NF-κB in the cardiac tissue of rats on an HFD.
3. Methods
3.1. Study Design
This experimental study was approved by the Ethics Committee in Biomedical Research of Ahvaz Islamic Azad University with the code IR.IAU.AHVAZ.REC.1402.040. Twenty-four male Wistar rats (8 - 10 weeks old; 200 - 250 g) were kept in standard transparent polycarbonate cages under a 12-hour light/dark cycle, at 22 ± 2°C, and with a relative humidity of 40 to 60%. The animals were divided into a control group (8 rats) and an HFD group (fed with food containing 60% fat and 25% fructose) (16 rats). After eight weeks, the rats treated with the HFD were further divided into the HFD group and the HFD + IAE group.
3.2. Exercise
To acclimate to the treadmill, the animals ran on the rodent treadmill for one week, five days per week (10 - 15 minutes; 5 - 10 m/minute). The maximum running speed of the rats was tested before beginning the training to determine the intensity of the training program and apply overload.
The IAE program was conducted for 6 weeks, with 5 sessions per week. The duration and speed increased from 10 m/minute for 10 minutes in the first week, to 10 m/minute (60% of maximum running speed) for 20 minutes in the second week, 14 - 15 m/minute (70% of maximum running speed) for 20 minutes in the third week, 14 - 15 m/minute for 30 minutes in the fourth week, and finally to 17 - 18 m/minute (80% of maximum running speed) for 30 minutes in the fifth and sixth weeks.
3.3. Tissue Samples
Forty-eight hours after the experiment, the animals were euthanized painlessly with ketamine/xylazine. After opening the chest, the heart tissue was dissected and promptly placed in a freezer at -80°C for storage until needed.
3.4. Real-time
RNA was isolated from the cardiac tissue using the RNeasy kit (Qiagen). To generate complementary DNA (cDNA), the RNA was reverse transcribed using a kit also provided by Qiagen. The PCR reaction mix consisted of cDNA, DEPC water, forward and reverse primers, and SYBR Green Master Mix (Qiagen). The qRT-PCR was performed using 45 cycles: 95°C for 50 seconds, 95°C for 30 seconds, and 60°C for 35 seconds. Relative gene expression was normalized using GAPDH. Data were analyzed using REST software (2009).
3.5. Data Analysis
A one-way analysis of variance (ANOVA) test (SPSS, version 21), followed by LSD or Kruskal-Wallis tests, was used to analyze the data. The significance level was set at less than 0.05.
4. Results
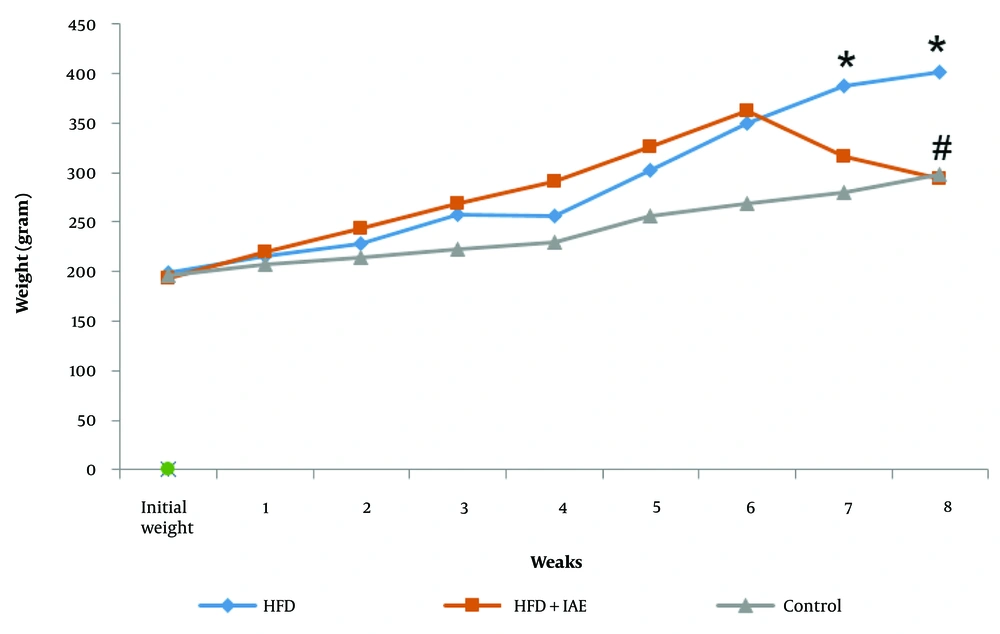
There was no significant difference between the initial weights of the rats in the research groups (P > 0.05). However, in the final week, the average weight gain in the HFD group was greater than that of the control group (P < 0.05). On the other hand, six weeks of IAE reduced the weight gain induced by the HFD. The average weight change in the HFD + IAE group was notably lower in the final weeks compared to the HFD group (P < 0.05, Figure 1).
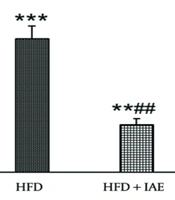
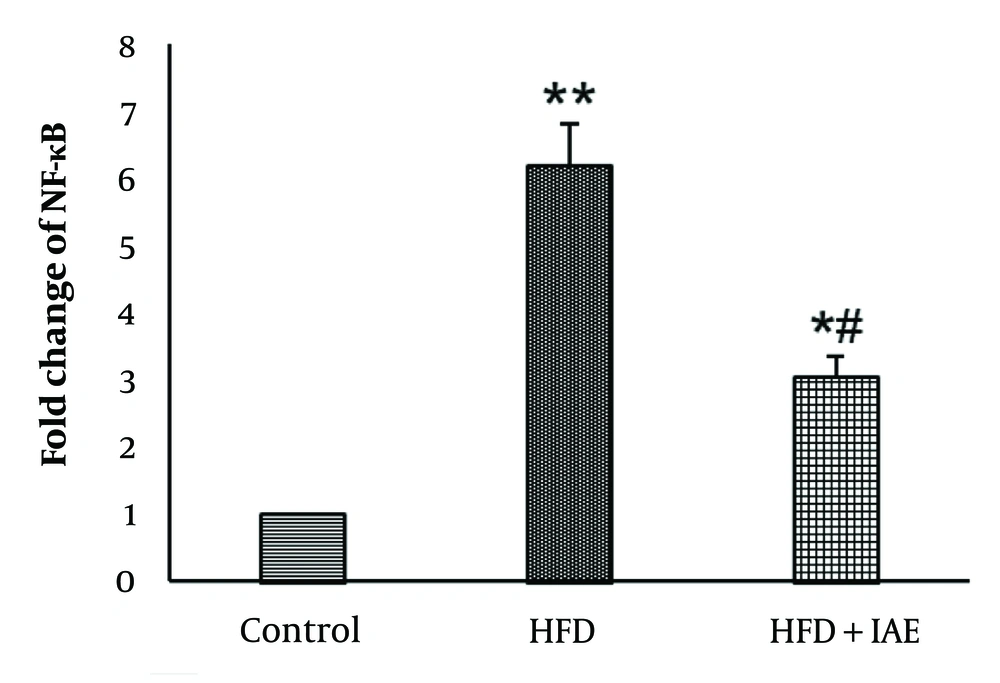
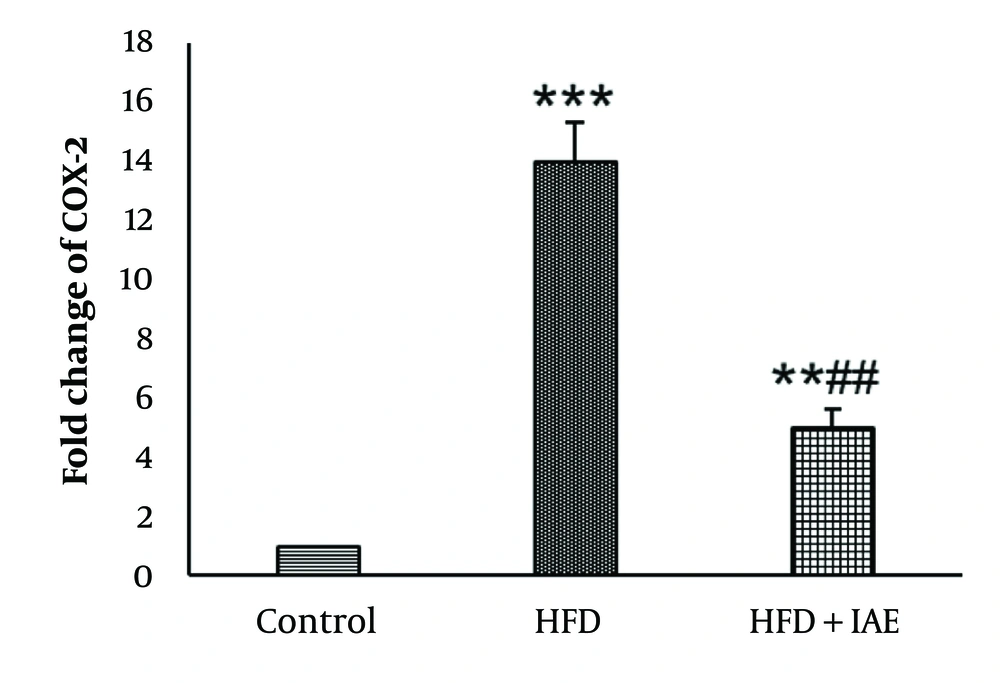
Figures 2 and 3 show that the highest levels of NF-κB and COX-2 were expressed in the HFD group, while the lowest levels were observed in the healthy control group. Additionally, the expression levels of NF-κB and COX-2 genes in the HFD + IAE group were significantly lower than in the HFD group (P < 0.01 and P < 0.05, respectively).
Data analysis revealed that the induction of obesity led to increased expression of NF-κB and COX-2 genes in the HFD group compared to the control group (P < 0.001 and P < 0.01, respectively).
There was a significant decrease in the expression of NF-κB and COX-2 genes in the HFD+IAE group compared to the HFD group (P < 0.05). No notable difference in the expression of NF-κB and COX-2 genes was found between the HFD+IAE group and the healthy control group (P > 0.05).
5. Discussion
The present study showed that an HFD increased the expression of NF-κB and COX-2 genes in heart tissue. In this context, Cortez et al. reported that an HFD increases the production of TNF-α, IL-6, and IL-1 in bone marrow mesenchymal stem cells by upregulating NF-κB (20). Nuclear factor Kappa B levels have been found to increase in obesity, and inhibiting it can lead to insulin resistance. Cyclooxygenase-2 is one of the major downstream molecular targets of NF-κB during the inflammatory process (21).
The enhanced NF-κB levels in the heart tissue of the HFD group were accompanied by an increase in the expression of the COX-2 gene. This finding indicates that the activation of the inflammation pathway is involved in obesity. The present study also showed that IAE decreased the expression of COX-2 and NF-κB genes in the heart tissue of rats fed an HFD. Padrao et al. demonstrated that 13 weeks of endurance training (5 days/week) downregulated NF-κB in the cardiac muscle of mice (22). Liu and Chang observed that moderate-intensity exercise inhibits NF-κB signaling and reduces NF-κB levels in diabetic rats (16).
Our research showed that IAE decreases the expression levels of COX-2 and NF-κB genes in the heart tissue of rats on an HFD. In contrast to our results, Kohpayeh et al. showed that low-intensity exercise and the consumption of crocin had no notable impact on NF-κB expression in mice (18). The intensity and duration of the training may explain the discrepancy between our findings and the study by Kohpayeh et al. In the current study, the duration and intensity of the exercise were equivalent to 60% to 80% of the maximum speed. The exercise sessions started at 10 minutes and increased to 30 minutes each, while in Kohpayeh et al.'s study, the exercise intensity was 50% to 55% of maximum running speed, starting at 19 minutes and continuing up to 47 minutes in each session (18).
Niksarasht et al. showed that eight weeks of aerobics had no notable impact on NF-κB in women with type 2 diabetes (23). This discrepancy may be due to differences in subject characteristics and sample size. Niksarasht et al. measured NF-κB levels in the serum of diabetic humans, while in our study, NF-κB levels were measured in the cardiac muscle of obese rats (23).
In another study, Firouzi-Niaki et al. demonstrated that six weeks of endurance training, consisting of five sessions per week lasting 50 minutes each at a speed of 14-18 m/second, along with the administration of taxol at a dosage of 60 mg/kg/day, decreased COX-2 levels in the liver tissue of rats (24). Shiraliet al. also showed that six weeks of swimming combined with the consumption of aloe vera extract reduced COX-2 activity in mice (17).
In the study by Saed-Mocheshi et al., aerobic training increased NF-κB protein levels in the prostate tissue of healthy mice but did not affect COX-2 protein levels (19). In their study, each training session lasted 45 minutes, and healthy mice were used, while in our study, the training sessions lasted 20 minutes and involved obese rats.
5.1. Conclusions
In conclusion, six weeks of IAE appears to reduce the activation of inflammatory pathways and lower tissue levels of enzymes involved in the inflammatory process caused by obesity. Future research should investigate the impact of IAE on pro-inflammatory and anti-inflammatory factors in obese subjects.