1. Background
Obesity is a serious health threat that affects all body organs and contributes significantly to the burden of non-communicable diseases such as type 2 diabetes and cardiovascular diseases (1). Activated protein kinase C (PKC) isoforms are a subgroup of serine/threonine kinases, divided into three subfamilies based on their ligand and cofactor structures. Protein kinase C interacts with PS, DAG/phorbol ester, and calcium (2). Ca2+ dependence is mediated by the C2 domain, while phorbol-ester binding requires two cysteine-rich zinc finger regions in the C1 domain. Protein kinase Cs are considered key enzymes in controlling cardiac cell growth and hypertrophy and play a critical role in signal transduction in the cardiovascular system.
In addition to PKC, a growing number of alternative sn-1,2-diacylglycerol (DAG) receptors, differing in structure, regulation, and function, have been discovered over the past decade (3). These receptors contribute to the various biological actions of DAG signaling. While diacylglycerol and its primary target, protein kinase C, regulate many important cellular responses, the molecular mechanisms controlling the specificity of DAG and PKC signaling are not fully understood (4). Phosphatidylinositol 4,5-biphosphate (PIP2) is also considered a major stimulator of lipid accumulation in cells, as it functions not only as a second messenger but also as an intermediate for producing additional lipid mediators (5).
Previous studies have well-documented that aerobic activity can reduce lipogenesis, decrease the incidence of obesity, and improve health profiles in obese patients (6, 7).
2. Objectives
Accordingly, the aim of the current study was to evaluate the efficacy of aerobic exercise on cardiac remodeling in experimental rat models of diet-induced obesity through the DAG, PKC, and PIP2 signaling pathways.
3. Methods
Thirty-two male Wistar rats were purchased from Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The animals were kept in standard conditions with free access to water and rodent food for one week. After acclimatization, the animals were randomly divided into four groups as follows:
(1) Control group: Given normal water and food under standard conditions for 6 weeks.
(2) Obesity model group: Fed a high-fat/fructose diet (60% fat and 25% fructose) for 6 weeks.
(3) Obesity model + exercise group: Fed a high-fat/fructose diet (60% fat and 25% fructose) combined with aerobic exercise for 6 weeks.
(4) Exercise group: Engaged in aerobic exercise for 6 weeks.
All stages of the research adhered to ethical principles for working with animals, in accordance with the ethical guidelines of the national institutes for the care and use of laboratory animals (Helsinki protocol 2006), and were approved by the Islamic Azad University of Ahvaz (Ethics code: IR.IAU.AHVAZ.REC.1402.121).
3.1. Aerobic Exercise Protocol
The endurance training protocol was performed over a period of 6 weeks. In this study, a moderate to intense endurance training protocol, with progressive intensity and duration, following the principle of gradual overload, was used. In the exercise groups, the rats were subjected to treadmill exercise, 5 sessions per week for 6 weeks. All training sessions were held at the end of the animals' sleep cycle, between 16:00 and 18:00 in the evening. The treadmill speeds were as follows:
- First week: Ten m/min for 10 minutes
- Second week: Ten m/min for 20 minutes
- Third week: Fifteen m/min for 20 minutes
- Fourth week: Fifteen m/min for 30 minutes
- Fifth and sixth weeks: Eighteen m/min for 30 minutes
To ensure that the animals reached a uniform adaptation, all training variables were kept constant during the final week (8).
3.2. Cardiac Hypertrophy Assessment
At the end of the study, all rats were anesthetized using a mixture of Ketamine (90 mg/kg) and Xylazine (9 mg/kg), and then intubated. Morphometric evaluations were conducted, including measurements of body mass, heart weight, lung weight, and tibia length. Subsequently, the following ratios were calculated for all groups: Heart weight/body weight (mg/g), heart weight/tibia length (mg/mm), and lung weight/tibia length (mg/mm) (9).
3.3. Real-time PCR Genes Expression Evaluation
To compare the gene expression levels of DAG, PKC, and PIP2, RNA was extracted from cardiac tissue samples, and its concentration was measured using absorbance. The extracted RNA was then used to synthesize cDNA via a cDNA Reverse Transcription Kit. Real-time PCR was subsequently performed using a master mix, following the manufacturer's instructions. Semi-log amplification curves were analyzed using the formula 2-∆∆CT, and the relative expression of each gene was normalized to that of GAPDH. The primer sequences used are provided below (Table 1).
| Gene | Forward | Reverse |
|---|---|---|
| DAG | GAGATTCAGAACGCCGTCCAG | GCGCCCCCTTTCTGCAAAACA |
| PKC | CTATCGCCGTCCACCTGTTTC | GCGCCCCCTTTCTGCAAAACA |
| PIP2 | TCTTGTCGTAGGGGCGATAC | GCCATCTTTGTCCACACCTT |
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
3.4. Statistical Analysis Methods
The obtained data are presented as means ± standard Errors. Data were analyzed using the ANOVA test with SPSS software (version 22). A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Aerobic Exercise Improved Cardiac Remodeling in Obese-Rats
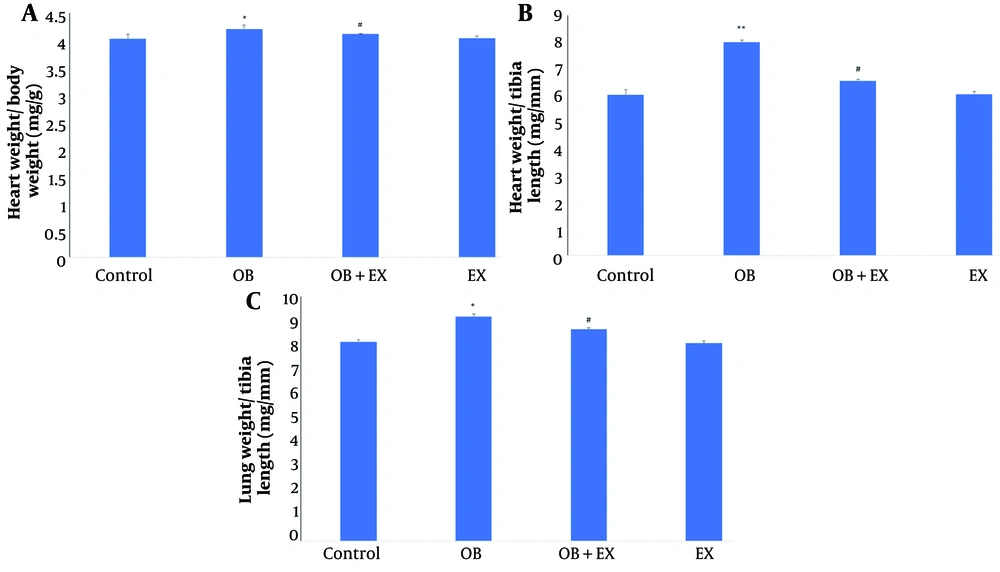
As shown in Figure 1, the morphometric measurements in the current study revealed that a high fructose/fat diet caused significant increases in heart weight/body weight (P < 0.05) (Figure 1A), heart weight/tibia length (P < 0.01) (Figure 1B), and lung weight/tibia length (P < 0.05) (Figure 1C) compared to the control rats. However, 6 weeks of exercise resulted in significant decreases in heart weight/body weight, heart weight/tibia length, and lung weight/tibia length indexes compared to the obese rats.
Comparison the A, heart weight/body weight (mg/g); B, heart weight/tibia length (mg/mm); and C, lung weight/tibia length (mg/mm) in all groups including: Control; OB, obesity model; OB + EX, obesity plus aerobic exercise group; and EX, aerobic exercise group. The data presents as Mean ± SEM. One-way ANOVA test. * Vs control and # Vs obesity group.
4.2. Aerobic Exercise Improved DAG/PKC/PIP2 Gene Expression in Obese-Rats
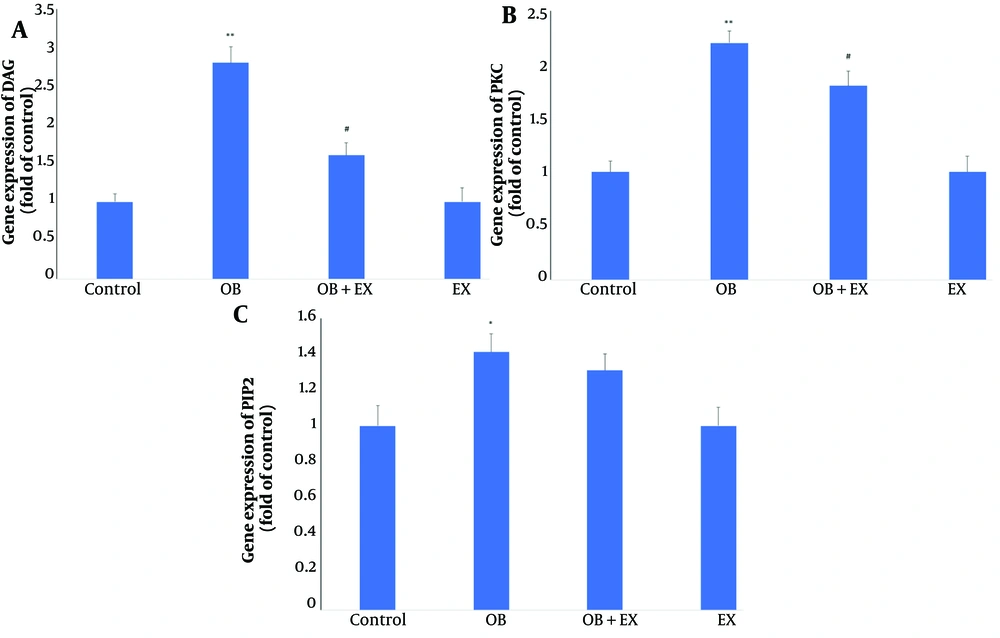
As shown in Figure 2, the real-time PCR evaluation in the current study demonstrated that a high fructose/fat diet caused significant increases in the gene expression levels of DAG (P < 0.01) (Figure 2A), PKC (P < 0.01) (Figure 2B), and PIP2 (P < 0.05) (Figure 2C) compared to the control rats. However, 6 weeks of exercise resulted in significant decreases in the gene expression levels of DAG (P < 0.05) and PKC (P < 0.05) compared to the obese rats. There were no significant changes in PIP2 gene expression in the exercise + obesity group compared to the obese rats.
Comparison the genes expression of A, diacylglycerol (DAG); B, protein kinase C (PKC); and C, phosphatidylinositol 4,5-biphosphate (PIP2) in all groups including: Control; OB, obesity model; OB + EX, obesity plus aerobic exercise group; and EX, aerobic exercise group. The data presents as Mean ± SEM. One-way ANOVA test. * Vs control and # Vs obesity group.
5. Discussion
The results of the current study documented that a high fat/fructose diet led to cardiac remodeling by increasing cardiac hypertrophy indices. Additionally, the gene expression levels of PKC, DAG, and PIP2 in the cardiac tissue of the obese group were elevated. However, 6 weeks of aerobic exercise improved cardiac morphometric indices by downregulating the PKC/DAG/PIP2 signaling pathway.
Obesity, characterized by excess body fat, is a global epidemic with serious consequences, including an increased risk of diseases and reduced life expectancy (10). The prevalence of obesity is rapidly rising worldwide and is now considered a major public health concern. According to the World Health Organization (WHO), there are more than 2 billion overweight adults globally, with over 600 million classified as obese (11). The global prevalence of obesity has tripled between 1975 and 2016. If this trend continues, it is estimated that nearly a third of the world’s adult population will be overweight, and more than 1 billion will be obese by 2025. Obesity imposes a heavy economic burden on health systems across many countries (12).
Studies show that obesity is an independent risk factor for cardiovascular diseases (CVD), including left ventricular hypertrophy, atherosclerosis, heart failure, and diabetes (13). Excess fat increases metabolic demands, leading to higher cardiac output and chronic overload, which causes structural changes in the left ventricle. The mass of fat tissue determines metabolic activity and may play a critical role in the structural changes of the heart (14). Changes in heart muscle structure can elevate the risk of atrial fibrillation and sudden cardiac death (15).
Aerobic exercise has been recommended as an effective and low-cost solution for the prevention and treatment of cardiovascular diseases (16). The use of aerobic exercises in experimental models with healthy rats has proven to be a valuable tool for identifying positive adaptations in cardiac function (17). However, the mechanisms through which regular exercise improves cardiac tissue structure, especially in obese subjects, remain poorly understood.
The results of the present study showed that the expression levels of PKC and DAG genes in the heart tissue of obese mice significantly increased compared to the control group. In line with these findings, Velazquez et al. demonstrated that a high-fat diet with 10% fructose reduces beta-oxidation and increases de novo lipogenesis. In liver examinations, it was reported that ceramide content increases in high-fat diets without fructose, while the high-fat diet group shows unchanged ceramide levels but an increase in diacylglycerols (18). Mechanisms linking DAG and ceramide accumulation include the activation of different protein kinase isoforms, such as PKC, or the inhibition of PKB signaling (19).
There is now substantial evidence supporting a potential role for protein kinase C beta (PKCβ) in energy homeostasis. Available data suggest a model in which activation of adipose PKCβ is one of the initial events that impairs mitochondrial function through interaction with p66shc, leading to lipid accumulation and dysfunction, with systemic consequences (20).
In a review conducted by Nijavan et al. in 2020, it was shown that stimulation of the PKC gene contributes to the development of obesity. Protein kinase C affects mitochondrial activity in adipose tissue, increasing lipid accumulation and causing systemic disorders due to disruptions in adipose tissue function. Obesity induces oxidative stress within adipose tissue, which dysregulates PKC activity (21). Therefore, controlling PKC expression and concentration may offer a novel approach to preventing high-fat diet-induced obesity and related diseases. Protein kinase C kinases and PKN-related families play multifaceted roles in cardiac development and the pathophysiology of various cardiovascular diseases. Protein kinase C has been reported to play a significant role in the development and progression of cardiac hypertrophy (22).
A high-fat diet leads to DAG accumulation and PKC activation. Aberrant lipid accumulation, including diacylglycerol or ceramide, has been demonstrated in cell culture systems and animal models, and is linked to insulin resistance. Mechanisms connecting muscle fiber DAG and ceramide accumulation to insulin resistance include activation of different PKC isoforms or inhibition of protein kinase B (PKB) signaling (23).
The results of this study also showed that aerobic exercise significantly altered PKC gene expression in the heart tissue of obese rats. Lira et al. reported that exercise training decreased cardiac free fatty acid (FFA) and DAG concentrations, consistent with the findings of the present study, which showed an increase in AMPK expression and a decrease in FOXO1 expression in the myocardium (24). These findings may also explain cardiovascular complications in athletes following a high-calorie diet.
Hydrolysis of phosphatidylinositol 4,5-biphosphate at the plasma membrane by phospholipase C is a key hormone-regulated intracellular signaling system. This system generates the diffusible second messenger IP3 and the membrane-bound messenger diacylglycerol. Although recent evidence suggests that exercise can suppress PIP2 activation, the current study found that aerobic exercise failed to reduce PIP2 gene expression in obese rats, which may be due to the short duration of the exercise regimen.
In summary, the findings of this study demonstrated that inducing obesity in rats with a high-fat and fructose-containing diet leads to cardiac hypertrophy, accompanied by increased expression of PKC, DAG, and PIP2 genes in cardiac tissue. However, the results also showed that aerobic exercise improved cardiac morphometric indices, which was associated with a decrease in PKC and DAG gene expression.