1. Background
According to the statistics released by the World Health Organization, breast cancer (BC) is currently the most frequent cancer diagnosed in females worldwide (1, 2). Despite the development of novel diagnostic procedures, relapse of the disease and resistance to therapy remain major challenges in the management of BC (3, 4). A review of the therapeutic effects of medicinal plants shows the usefulness of these compounds in the prevention or treatment of diseases.
Juglans Regia L., or the Persian walnut, is a native tree of Central Asia and belongs to the Juglandaceae family (Juglans genus). Walnuts from different areas of the world differ in various phenotypic features, including the properties of the fruit, shell, and kernel, which are defined by their genetic characteristics. Due to its high nutritional value, walnuts have occupied an important place in human nutrition since ancient times. Additionally, some Juglans species have been applied in traditional medicine for cancer treatment; for example, the green husk has shown therapeutic properties against malignancies in China, Korea, and Japan (5). Polyphenols are the main compounds of the walnut green husk. Among them, caffeic acid, ferulic acid, chlorogenic acid, sinapic acid, juglone, gallic acid, protocatechuic acid, syringic acid, vanillic acid, catechin, epicatechin, myricetin, an alpha-tetralone derivative (regiolone), di-aryl heptanoids juglanin A and B, and rhoiptelol have the highest concentration in the walnut green husk (6, 7).
It has been shown that the methanolic extract of Juglans green husk inhibits MCF-7 proliferation, a BC cell line. This compound exhibits anti-tumorigenic properties at doses of 0.5 mg/mL and 1 mg/mL, causing 93% and 86% of cells to die, respectively (8). Other extracts of J. Regia L. green husk, like dichloromethane, were also studied to explore a possible anti-proliferative effect on several human cancer cells, including breast, colon, cervix adenocarcinoma, and leukemic cells. Notably, the results indicated a highly potent anti-tumor effect for some compounds (9). Additionally, when examined in the MDA-MB-231 cells, root bark extract of this plant shows high apoptotic and anti-proliferative properties (10). Furthermore, it has been revealed that the anti-cancer property of J. Regia could be seen in several leaf extracts, such as total extract, hexane, chloroform, ethyl acetate, and methanol extracts. The results showed that all fractions could inhibit the growth of the BHY, MCF-7, and HT-29 cell lines, with the chloroform fraction showing the highest cytotoxicity, particularly in MCF-7 and BHY cells (11).
In addition to MCF-7 BC cells, crude extract showed therapeutic effects on other cancer cells. Another study observed the ability of aqueous walnut milk to prevent the progression of breast and prostate tumor cells (12). It has been suggested that J. Regia L. green husk extract promotes apoptosis in the PC-3 cell line (a prostate cancer cell lineage) by over-expressing associated genes such as Bax, Caspase 8, and Caspase 3, while reducing B-cell lymphoma 2 (Bcl-2) gene expression (13). Regarding HL-60 cells, it has been revealed that green husk extract exerts its anti-proliferative effect via induction of apoptosis and decreasing mitochondrial membrane potential (14). Naphthoquinones like juglone (5-hydroxy-1, 4-naphthoquinone), which can be found in almost all parts of various Juglans species, have shown medicinal properties against a wide range of diseases, including malignancies, gastrointestinal disorders, acne, allergies, intestinal parasitosis, and fungal, bacterial, and viral infections (15).
Despite numerous investigations performed on the cytotoxic properties of walnut green husk extract, a paucity of studies has been conducted on its anti-proliferative role in BC cells.
2. Objectives
In this study, we compared the cytotoxic and anti-proliferative properties of methanolic extract of the native Juglans green fruit pericarp from the Kurdistan area on BC cell lines.
3. Methods
3.1. Preparation of Juglans Green Fruit Pericarp Extract
Samples of Juglans green fruit pericarp were collected from gardens in Sanandaj, Kurdistan, Iran, and kept at -18ºC for further investigations. Then, 20 g of fresh Juglans green fruit pericarp was ground and soaked in 200 mL of methanol and kept at room temperature for 3 days. The solvent was then filtered, and the extract was dried using a rotary evaporator (Heidolph, Germany) at 60°C (16).
3.2. Sample Preparation
To prepare samples, 0.1 g of the extract was dissolved in 1 mL methanol and then diluted with RPMI-1640 culture media to prepare concentrations of 125, 250, and 500 μg/mL. The treatment solutions were freshly prepared before conducting the experiments (16).
3.3. Gas Chromatography/Mass Spectrometry
The methanol solution of Juglans green fruit pericarp was evaluated using an Agilent 7890B/5977A GC/MSD device (Agilent, USA). The gas chromatography/mass spectrometry (GC/MS) equipment included an HP-5ms column (30 m × 0.25 mm × 0.25 μm) interfaced with a quadrupole mass detector. The data were obtained and managed by a computer equipped with the Wiley 7n.l library. The chromatographic and mass spectrometric separation and analysis settings were as follows: Oven temperature 60°C (1 min), 60 to 250°C (5°C/min), 250°C (38 min); injector temperature, 250°C; injection volume, 0.1 μL; split ratio, 1: 50; Helium as the carrier gas at 1 mL/min; potential ionization, 70 eV; current ionization, 150 μA; ion source temperature, 250°C; mass range, 35 - 465 m/z. The retention indices (RI) and mass spectra of each component were compared with validated samples and previous literature to determine distinct compounds. The corresponding amount for each compound was calculated via the area percentage (16).
3.4. Cell Culture
Hormone receptor-positive (MCF-7) and hormone receptor-negative (MDA-MB-231) BC cell lines were obtained from the International Centre for Genetic Engineering and Biotechnology (ICGEB) (Tehran, Iran) and grown in RPMI-1640 medium (Invitrogen, Carlsbad, CA) containing 10% FBS (Gibco BRL). The cells were kept in a 37°C incubator with 5% CO₂ (17).
3.5. Proliferation Assay
The toxic effect of the extract was assessed using a commercial MTT kit (Kiazist) according to the manufacturer's instructions. Briefly, cancer cells were seeded in a 96-well plate at a concentration of 5000 cells per well and treated with the methanol extract (125, 250, and 500 μg/mL) for 72 hours. Treatment with the solvent alone and an untreated experiment group served as negative controls, while paclitaxel (1 μM/mL) was used as a positive control. The optical density of each experimental set was measured photometrically at 570 nm (17).
3.6. Cell Cycle Analysis
The two BC cell lines (MCF-7 and MDA-MB-231) were grown in 6-well plates until reaching 80 - 90% confluency and then treated with methanol extract at concentrations of 333.9 μg/mL for MDA-MB-231 and 176.8 μg/mL for MCF-7. The cells were incubated for 72 hours. A similar procedure was performed for controls. The cells were then fixed with 70% ethanol for 1 hour at 4°C and incubated with propidium iodide (PI) (Sigma-Aldrich) for 1 hour at room temperature. Cell cycle evaluation was performed using a BD FACSCalibur flow cytometer (BD Biosciences, Mountain View, CA). The number of events reported in each assessment was twenty thousand. FlowJo software (TreeStar, Inc., San Carlos, California, USA) was used to measure the frequency of cells in each cell cycle phase (17).
3.7. Colony Formation Assay
To evaluate the potential of the BC cells to form colonies, they were cultured at a density of 500 cells per well and treated with methanol extract (333.9 μg/mL for MDA-MB-231 and 176.9 μg/mL for MCF-7) for 14 days. The cells were then washed with PBS and fixed with a 3: 1 methanol: Acetic acid solvent. The fixed cells were stained with 0.04% crystal violet (Merck, CI 42555) for 40 minutes. The stained colonies were washed with PBS and counted.
3.8. Statistical Analysis
Data analysis was conducted using SPSS version 16 (SPSS Inc., Chicago, USA). The results were presented as means ± standard deviation. Differences between two groups were examined using a t-test, while one-way ANOVA was employed for comparisons between multiple groups. The Spearman correlation test was used to determine the correlation between variables. A P-value below 0.05 was considered statistically significant. All experiments were replicated three times.
4. Results
4.1. Chemical Composition of the MetOH Extract of Juglans Regia L. Green Fruit Pericarp
After preparing the methanol extract of J. Regia L. green fruit pericarp, we first examined the main constituents of the extract using GC/MS. Our results showed that the methanol extract consists of 16 volatile compounds. The main constituents of the extract were identified as 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl (28.378%), 7-Oxabicyclo [4.1.0] heptan-2-one, 6-methyl-3-(1-methylethyl) (25.526%), and Ascaridole epoxide (12.944%). The chemical composition and amounts of these compounds in different extracts are illustrated in Table 1.
| Compound | Concentration (%) | Retention Time (min) | Molecular Formula |
|---|---|---|---|
| 1,3-Oxathiane, 2-(1- methylethyl) | 1.101 | 6.672 | C7H14OS |
| 2-Heptanol, acetate | 0.962 | 7.532 | C9H18O2 |
| 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | 3.265 | 8.169 | C6H8O4 |
| 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | 6.263 | 8.311 | C6H8O4 |
| L-Glucose | 2.486 | 8.598 | C6H12O6 |
| Ethanethioic acid, S-(tetrahydro-2H-pyran-3-yl) ester | 1.994 | 9.473 | C7H12O2S |
| Alpha-l-rhamnopyranose | 3.729 | 10.104 | C6H12O5 |
| 9-Oxabicyclo [3.3.1] nonan-2-one, 6-hydroxy | 1.206 | 11.164 | C8H12O3 |
| Alpha-D-Glucopyranoside, O-. alpha. -D-glucopyranosyl-(1. fwdarw.3)-. beta. -D-fructofuranosyl | 3.450 | 11.853 | C18H32O16 |
| Alpha-D-Glucopyranoside, O-alpha-D-glucopyranosyl-(1. fwdarw.3)-. beta. -D-fructofuranosyl | 1.288 | 12.013 | C18H32O16 |
| Alpha-D-Galactopyranoside, methyl 2-(acetylamino)-2-deoxy-6-O-methyl | 0.679 | 12.895 | C10H19NO6 |
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl | 28.378 | 13.421 | C6H8O4 |
| Bicyclo[3.2.1]oct-6-ene-6,8-dimethanol, 1,7-dimethyl-4-isopropyl-, bis(3,5-dinitrobenzoate) | 5.810 | 15.329 | C29H30N4O12 |
| Cyclohexanone, 4-ethoxy | 0.919 | 15.928 | C8H14O2 |
| Ascaridole epoxide | 12.944 | 16.761 | C10H16O3 |
| 7-Oxabicyclo [4.1.0] heptan-2-one, 6-methyl-3-(1-methylethyl) | 25.526 | 18.111 | C10H16O2 |
Chemical and Volatile Components of the MetOH Extract of Juglans Regia L. Green Fruit Pericarp
4.2. Proliferation Analysis
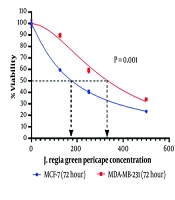
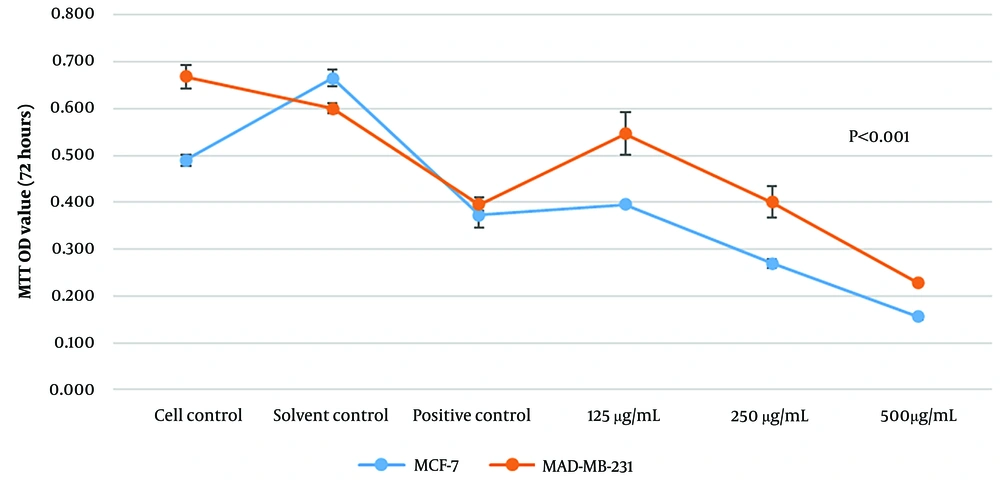
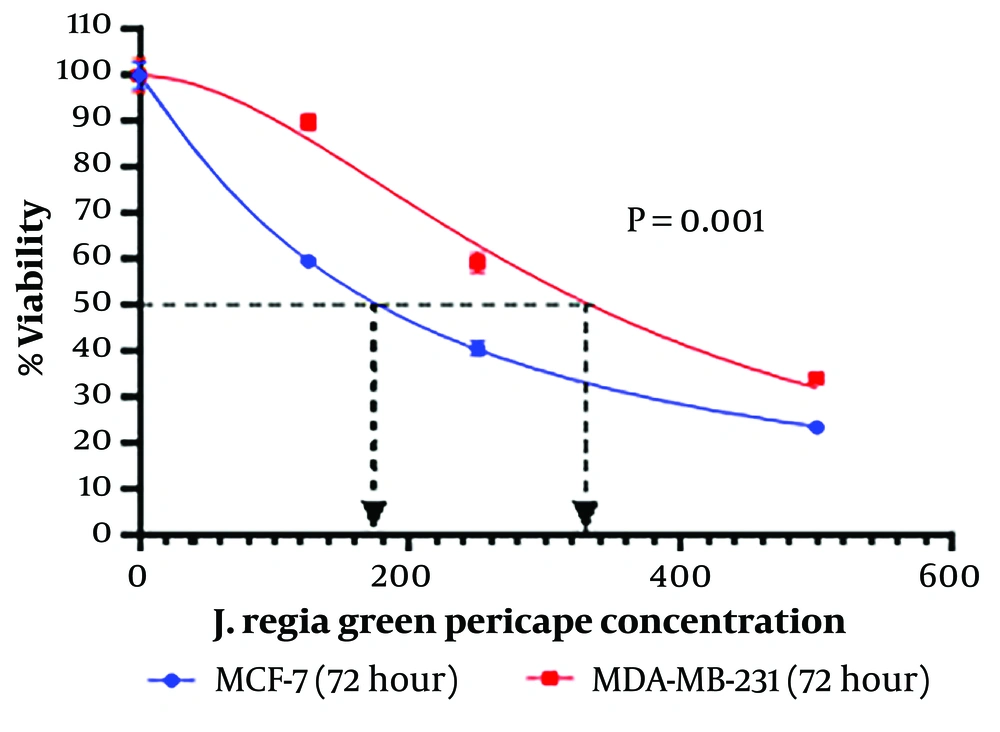
Two BC cell lines (MCF-7 and MDA-MB-231) were treated with different concentrations of the methanol extract of J. Regia L. green fruit pericarp (125, 250, and 500 μg/mL) for three consecutive days. Our results revealed that after 72 hours, the viability of both MCF-7 and MDA-MB-231 cell lines was significantly reduced following treatment with the methanol extract (Figure 1). We next determined the concentration that caused 50% cell growth inhibition (IC50). According to our results, the IC50 of J. Regia L. green fruit pericarp extract was 333.9 μg/mL for MDA-MB-231 cells and 176.9 μg/mL for MCF-7 cells (Figure 2).
The proliferation rate of the breast cancer (BC) cells was analysed using MTT assay. The MTT assay revealed that the Juglans Regia L. green fruit pericarpe methanolic extract had significant effect on MCF-7 cell line viability. Also, the proliferation of MDA-MB-231 cell line were significantly inhibited after treatment with methanol extract of J. regia L. green fruit pericarpe compared to control (concentrations are expressed as μg/mL).
4.3. Juglans Regia L. Green Fruit Pericarp Methanol Extract Hinders Cell Cycle in the Breast Cancer Cell Lines
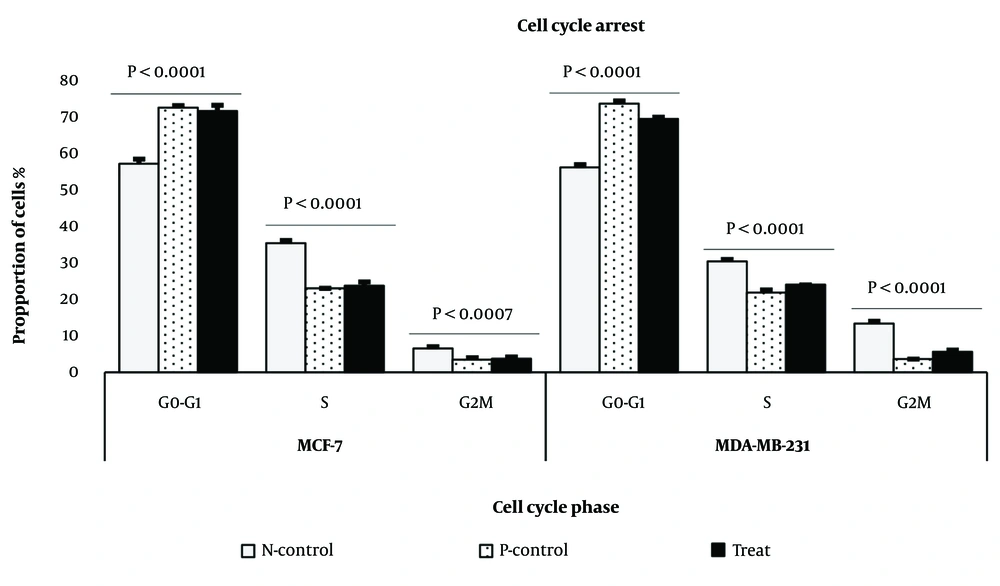
We further analyzed the effect of the methanol extract of J. Regia L. green fruit pericarp on the MCF-7 and MDA-MB-231 cell lines. We observed that G0/G1 phase arrest was significantly increased following treatment with the methanol extract in both BC cell lines, while the S phase was more prominent in the controls (Figure 3) (P < 0.001).
The methanol extract of Juglans Regia L. green fruit pericarpe inhibited cell growth by suppressing cycle phase transition from G0/G1 to S in MCF-7 and MDA-MB-231 breast cancer (BC) cell lines as compared to control. The doses of 176.9 μg/mL (for MCF-7) and 333.9 μg/mL (for MAD-MB-231) significantly enhanced cell cycle arrest in G0/G1 phase when compared to control group in both BC cell lines.
4.4. Colony Formation Ability
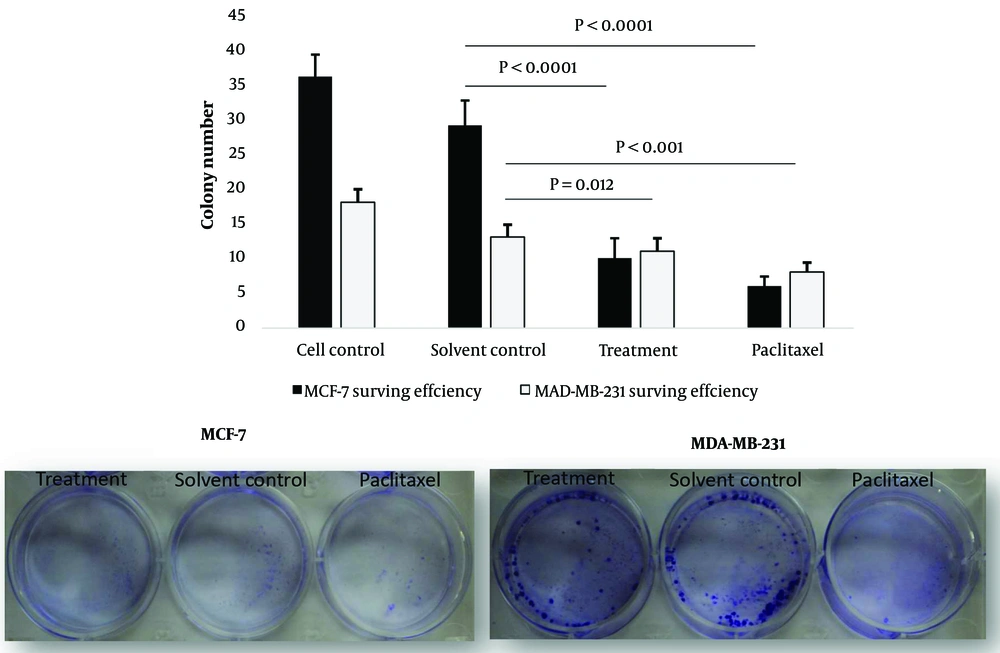
The BC cells were treated with the IC50 dose of the methanol extract and examined for changes in their ability to form colonies. We observed that after treatment with Juglans green fruit pericarp methanol extract, colony formation in MCF-7 and MDA-MB-231 cell lines was remarkably reduced compared to the negative control group (Figure 4). Interestingly, we revealed that the colony number of the MCF-7 cell lines was lower than that of MDA-MB-231 cell lines after treatment with the methanol extract.
The formation of MCF-7 and MDA-MB-231 colonies were determined by colony formation assay: Decrease in the formation of breast cancer (BC) colonies post treatment with the methanolic extract of the Juglans Regia L. green fruit pericarpe is more significant in the MCF-7 cell line than MDA-MB-231 cells. Treatment with methanol extract of J. regia L. green fruit pericarpe significantly decreased the number of colonies formed by MAD-MB-231 and MCF-7 cells compared to non-treated control.
5. Discussion
To improve responses to cancer treatment strategies and reduce side effects, there has been increasing attention on the use of herbal medicine as a complementary therapy. Juglans Regia L. green fruit pericarp methanol extract demonstrated a high anti-proliferative effect against cancer cells. In this study, we confirmed that the methanol extract of J. Regia L. green fruit pericarp suppresses the proliferation of two BC cell lines. Our findings also revealed that the cytotoxicity of J. Regia L. green fruit pericarp methanol extract was significantly lower in the MDA-MB-231 cell line compared to MCF-7, likely due to the specific characteristics of the MDA-MB-231 cell line.
In addition to the extraction protocol, J. Regia L. green fruit pericarp was observed to contain various bioactive compounds. Our study found that the methanol (MetOH) extract was rich in phenolic and complex compounds. The main constituents of the MetOH extract were identified as 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl (28.378%), 7-Oxabicyclo [4.1.0] heptan-2-one, 6-methyl-3-(1-methylethyl) (25.526%), and Ascaridole epoxide (12.944%). Our results demonstrated that concentrations of 333.9 μg/mL (for MDA-MB-231 cells) and 176.9 μg/mL (for MCF-7 cells) of the MetOH extract from J. Regia L. green fruit pericarp reduced the viability of BC cells. Consistent with previous studies (18, 19), our findings confirmed the anti-tumor effect of J. Regia L. green fruit pericarp extract on MCF-7 cell lines.
For the first time, our investigation revealed the anti-tumorigenic potential of J. Regia L. green fruit pericarp extract on metastatic MDA-MB-231 BC cells. MCF-7 is a luminal a BC cell line that expresses hormone receptors (ER and PR positive), is HER2-negative, and has low Ki-67 expression, leading to slower progression. These characteristics make luminal A the lowest-grade BC subtype with the best prognosis. In contrast, MDA-MB-231 is a triple-negative BC (TNBC) subtype (Claudin-low) known for its metastatic behavior and intermediate response to chemotherapy (20). Interestingly, we showed that J. Regia L. green fruit pericarp extract may suppress the growth of TNBC cells. The methanol extract's main components were phenolic compounds, and the anti-tumorigenic effects on BC cells may be attributed to these compounds. Furthermore, our flow cytometry results revealed an increase in cell cycle arrest after treating BC cell lines with the methanol extract of Juglans green fruit pericarp.
The results from previous studies regarding the dose that shows the highest anti-cancer effect are quite contradictory. Soto-Maldonado et al. (14) demonstrated that green husk extract of J. Regia L. generates cytotoxicity against HL-60 cells by decreasing mitochondrial membrane potential and inducing early apoptosis. A low concentration (1 μM) was safe for normal cells, while both normal and malignant cell viability decreased at higher concentrations. In a study by Alshatwi et al. (10), they showed that the anti-proliferative and apoptotic properties of green husk extracts are dose- and time-dependent. This involved DNA fragmentation and modulation of gene expression. An over-expression of Bax, Caspase-3, and TP53 genes and a down-regulation of Bcl2 expression post-treatment with extracts in prostate cancer cells revealed a potential anti-cancer property for J. Regia green husks, which can induce apoptosis in prostate carcinoma cells. This part of the plant was introduced as a possible drug candidate for cancer treatment.
In summary, our results demonstrated the anti-proliferative properties of methanol (MetOH) extract on BC cell lines. Our study revealed that the MetOH extract of J. Regia inhibited the growth of BC cells, and this cytotoxicity was mediated by increasing G0/G1 cell cycle arrest. Therefore, the use of J. Regia could be considered as a potential complementary medicine in future in vivo studies.