1. Background
Gastric cancer (GC), the fifth most common cancer, is the third leading cause of cancer-related death worldwide. In 2018, nearly 1.033 million new GC cases were diagnosed, and approximately 783,000 deaths occurred, according to GLOBOCAN estimates (1). The Cancer Genome Atlas (TCGA) project has identified four molecular subtypes of GC: Microsatellite instability (MSI), chromosomal instability (CIN), genomically stable (GS), and Epstein–Barr virus (EBV). The CIN subtype is commonly found in the esophago-gastric junction/cardia and accounts for at least 50% of GCs (2). Long non-coding RNA (lncRNA) molecules are involved in cancer and metastasis. The lncRNAs are longer than 200 nucleotides and do not translate into proteins (3). These molecules can originate from various genomic locations, including 3'- and 5'-UTRs, enhancer sequences, exon sequences, promoters, introns, and intergenic regions. The lncRNAs have various functions, including regulating gene transcription, epigenetic modification, translation, and post-transcriptional changes (4). Aberrant expression or dysfunction of lncRNAs causes many diseases. Many lncRNAs, with their tumor suppressor or carcinogenic effects in cancers, show irregular expression and can control cancer cell proliferation, angiogenesis, metastasis, or invasion. Due to their structural flexibility, such molecules can act as traps, signals, guides, or molecular scaffolding affecting metastasis (3, 5). The lncRNAs have been identified as novel biomarkers for the diagnosis, staging, and prognosis of GC (6). The lncRNAs have tissue-specific expression patterns and can be used to classify tumor subtypes or predict drug therapeutic effects (7, 8). Additionally, lncRNA polymorphisms (SNPs) are strongly associated with disease risk and significantly influence lncRNA expression levels (9-11).
The incidence of GC in Northwestern (e.g., Ardabil) and Northern (Caspian Sea littoral) regions of Iran has been reported to be considerably high [age-standardized incidence rates (ASRs) > 20/100,000]. In contrast, the Southern provinces, including Khuzestan, Kerman, and Yazd, exhibit much lower rates, with ASRs < 10/100,000. Despite these disparities, the specific contributions of bacterial and host factors, as well as geographic and ethnic differences in the incidence of GC in Iran, remain unclear. Ardabil exhibits the highest incidence rates of GC, with ASRs of 51.8 per 100,000 for males and 24.9 per 100,000 for females, especially concerning cardia GC, in both Iran and Central Asia (12-15).
2. Objectives
In light of this, we assessed the expression levels of long intergenic non-protein coding RNA 659 (LINC00659-ENSG0000022805), known for its oncogenic implications in colorectal cancer (CRC) (16), among GC patients in Ardabil to examine its potential as a diagnostic biomarker.
3. Methods
3.1. Study Cases
Forty-one tumor samples from GC patients, as well as 41 samples from corresponding adjacent non-cancerous tissues (ANCTs), were collected (n = 82) from GC patients who underwent endoscopy at Imam Khomeini Hospital of Ardabil from October 2017 to February 2020. The resection margin, defined as the distance from the tumor border to the adjacent tissue, was 1.5 to 2 cm. The examined cryopreserved samples were freshly sliced and stored in liquid nitrogen to maintain their morphological integrity until use. Tissue specimens were assessed via standard histopathological assessments. Histopathological assessments were conducted in accordance with the revised Sydney classification system (17). Additionally, Lauren's classification was employed to identify the histomorphological characteristics of the tumors, categorizing them as intestinal-type or diffuse-type adenocarcinomas, squamous cell carcinoma, and mucinous gastric carcinoma (18). Furthermore, the tumors were classified by their anatomical location based on the 10th Revision of the International Classification of Diseases, specifically C16.0 for cardia and C16.1 - C16.9 for non-cardia, which includes overlapping and unspecified subsites (https://apps.who.int/iris/handle/10665/42980). Patients subjected to radiation therapy, chemotherapy, or immunotherapy were not included. Informed consent was signed by GC patients. This study was approved under the ethical approval code of IR.ARUMS.REC.1396.160. All tissues were stored at -80°C.
3.2. RNA Extraction, cDNA Synthesis, and Real-time Reverse Transcriptase PCR
The TRIZOL Kit (Invitrogen) was used to separate total RNAs from the samples based on the manufacturer’s protocol. The quantity and quality of the extracted RNAs were assessed using a NanoDrop2000c spectrophotometer (Thermo Fisher). The cDNA was synthesized with a Takara kit (TaKaRa, Dalian, China). A total of 1 µg of RNA was reverse transcribed in a final volume of 20 μL with random primers. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to quantify lncRNA as an internal reference. Quantitative real-time PCR reactions were carried out with the SYBR Green Master Mix (Takara). A Rotor-Gene 6000 Corbett real-time PCR System was used to compare the relative lncRNA expression level between non-tumoral and tumoral samples. There was a non-template negative control for every run. The following gene-specific primers were used for LINC00659 and GAPDH: LINC00659 - forward /5′-ACCCTGAAGGACCATATCCA-3′, LINC00659 - reverse /5′-GGCTCGGCTGTGTCTCAAG-3′, GAPDH - forward /5′-TGCACCACCAACTGCTTA-3′, and GAPDH - reverse /5′-GGATGCAGGGATGATGTTC-3′. Relative fold-change was calculated using the 2−ΔΔCt value. Experiments were repeated in triplicate.
3.3. Statistical Analysis
Relative quantification of the target gene expression was calculated using a two-sided Pair-Wise Fixed Reallocation Randomization Test by Relative Quantification Software Tool (REST 2009) software. Fold change was determined to detect gene expression differences between tumor tissues and ANCTs. The chi-square test (χ2) was employed for assessing the correlation between LINC00659 gene expression and clinicopathological characteristics. P-values < 0.05 were considered significant.
4. Results
Our study examined LINC00659 expression levels in GC tissues compared with non-tumor tissues (n = 82) through real-time RT-PCR. The average age of cases was 66.02 ± 9.2 years (range, 42 to 83 years). Additionally, 74.3% of them were males. Furthermore, 63.4% of tumors were found in the cardia subsite, 9.8% in both the cardia and non-cardia subsites, and 26.8% in the non-cardia subsite. Histologically, 42.5% of them had the diffuse type, 42.5% had the intestinal type, and 15% had other GC types. For 25% of patients, there was a positive family history of GC (first or second degree). The LINC00659 relative expression level was not significantly different between tumor samples compared to controls [Fold change (mean ± SE): 0.57 ± 0.13, P = 0.33; Figure 1A].
A and B, calculations of fold change (mean ± SE) in expression levels between the normal and tumor paired samples for all samples and for anatomical sites and histopathological types of the tumors. P-values for total, cardia gastric cancer (GC), non-cardia GC, cardia/non-cardia GC, intestinal- and diffuse-type GC [vs. adjacent non-cancerous tissue (ANCTs)] were 0.33, 0.43, 0.36, 0.74, 0.41, and 0.58, respectively.
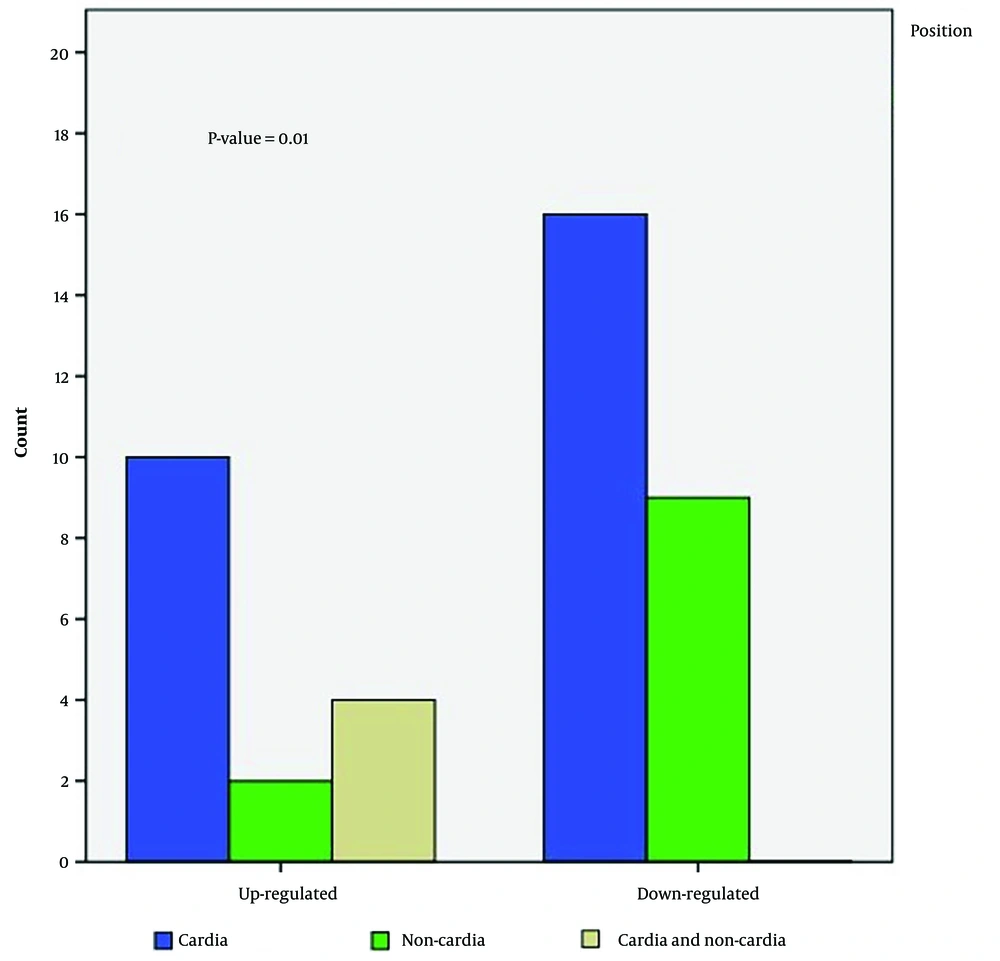
The relative expression level was also not significantly different (between tumors vs. ANCTs) when the analysis was performed based on anatomical sites and histopathological types of the tumors. The fold change (mean ± SE) was 0.57 ± 0.32 and 0.41 ± 0.18 for cardia and non-cardia subsites, respectively, and 0.46 ± 0.24 and 0.63 ± 0.16 for intestinal-type GC and diffuse-type GC, respectively (Figure 1A and B). In contrast, we found a significant association between LINC00659 expression levels and tumor origin after classifying patients into up- or down-regulation groups (P = 0.01; Table 1; Figure 2). In the up-regulation (vs. down-regulation) group, 38.5% (vs. 61.5%) of tumors were found in the cardia subsite, 18.2% (vs. 81.8%) in the non-cardia subsite, and 100% (vs. 0%) in both the cardia and non-cardia subsites. According to histological characteristics, in the up-regulation (vs. down-regulation) group, 41.2% (vs. 58.8%) had the diffuse type, 41.2% (vs. 58.8%) had the intestinal type, and 33.3% (vs. 66.7%) had other GC types. Such an association was not significant for other clinicopathological characteristics, such as age, pathology, gender, and family history of GC (P > 0.05).
| Variables | LINC00659 Up-Regulation | LINC00659 Down-Regulation | P-Value |
|---|---|---|---|
| Age | 0.09 | ||
| < 60 | 1 (12.5) | 7 (87.5) | |
| ≥ 60 | 13 (44.8) | 16 (55.2) | |
| Sex | 1 | ||
| Female | 10 (38.5) | 16 (61.5) | |
| Male | 3 (33.3) | 6 (66.7) | |
| Tumor origin | 0.01 | ||
| Cardia | 10 (38.5) | 16 (61.5) | |
| Cardia and non-cardia | 4 (100) | 0 (0) | |
| Non-cardia | 2 (18.2) | 9 (81.8) | |
| Pathology | 0.94 | ||
| Intestinal | 7 (41.2) | 10 (58.8) | |
| Diffuse | 7 (41.2) | 10 (58.8) | |
| Other | 2 (33.3) | 4 (66.7) | |
| Family history | 0.85 | ||
| Positive | 4 (40) | 6 (60) | |
| Negative | 11 (36.7) | 19 (63.3) |
a Values are expressed as No. (%).
b Up- and down-regulation of the gene have been defined based on the relative expression of the gene in malignant samples versus adjacent non-cancerous tissues.
5. Discussion
The emergence of high-throughput RNA sequencing (RNAseq) has led to the identification of thousands of unknown lncRNAs, whose improper expression is closely associated with cancer development and initiation. Dysregulation of lncRNAs is closely related to tumorigenesis, metastasis, diagnosis, or prognosis of GC. Recently, several lncRNA-associated GCs have been detected. Nonetheless, the underlying mechanisms regulating GC progression remain largely unknown. In the study by Wang et al., it was shown that LINC00659 levels were significantly elevated in GC. SP1 activated the up-regulation of LINC00659 in GC. There was an association between higher levels of LINC00659 and TNM stage, lymphatic metastasis, and poorer prognosis. Overexpression of LINC00659 also resulted in a reduction of AQP3, which was suppressed by miR-370 mimics. LINC00659 appears to act as a molecular sponge for miR-370, modulating AQP3 expression as a tumor promoter in GC. In an m6A-YTHDF2-dependent manner, LINC00659 upregulated JAK1 mRNA by facilitating ALKBH5 binding. The JAK1 axis was disrupted when ALKBH5 or LINC00659 were silenced. As JAK1 was up-regulated in GC, the JAK1/STAT3 pathway was activated (19).
In the study by Wang et al., it was shown that LINC00659 levels were clearly elevated in GC. SP1 activated the up-regulation of LINC00659 in GC. There was an association between higher levels of LINC00659 and TNM stage, lymphatic metastasis, and poorer prognosis. Overexpression of LINC00659 also resulted in a reduction of AQP3, which was suppressed by miR-370 mimics. LINC00659 appears to act as a molecular sponge for miR-370, modulating AQP3 expression as a tumor promoter in GC (19). In an m6A-YTHDF2-dependent manner, LINC00659 upregulated JAK1 mRNA by facilitating ALKBH5 binding. The JAK1 axis was disrupted when ALKBH5 or LINC00659 were silenced. As JAK1 was up-regulated in GC, the JAK1/STAT3 pathway was activated (20). LINC00659 derived from cancer-associated fibroblasts (CAFs) promotes CRC cell proliferation, invasion, and migration via the miR-342-3p/ANXA2 axis (21). Zhang et al. revealed that LINC00659 binds directly to the polypyrimidine tract-binding protein (PTBP1). By knocking down LINC00659 expression, PTBP1 binding was inhibited under H. pylori infection. LINC00659 knockdown reduced gastric epithelial cell senescence induced by H. pylori infection. It also suppressed interleukin-6 and IL-8 secretion through NF-κB p65 phosphorylation reduction (22). Sheng et al. indicated that LINC00659 expression levels were markedly up-regulated in GC patients. Elevated levels of LINC00659 were associated with advanced tumor stage and unfavorable prognosis for GC patients. Also, up-regulated LINC00659 expression could promote GC cell invasion. Bioinformatics studies indicated IQ motif containing GTPase activating protein 3 and matrix metalloproteinase 15 as possible downstream targets of LINC00659 associated with tumor metastasis. However, the exact underlying mechanism needs further exploration (23). Sheng et al. reported an expression level of LINC00659 in GC, which can be due to the promotion of cell invasion and regulation of cell proliferation. SUZ12 is a transcription factor and regulates LINC00659. Also, LINC00659 regulates cell cycle and GC invasion through an increase in SUZ12 expression (23). LINC00659 up-regulation was linked with poor survival in CRC patients. LINC00659 was found to have a significant co-expression with cycle-related genes in CRC. Silencing of LINC00659 expression caused cell growth inhibition and induced apoptosis, probably through suppression of AKT-PI3K signaling in colon cancer (16). On the contrary, in our study, although LINC00659 was down-regulated in more GC samples than controls (expression ratio = 0.57), the difference did not reach statistical significance. After classifying patients into up- or down-regulation groups, a significant association was found between LINC00659 expression levels and tumor site (P < 0.05). No significant association between LINC00659 expression levels and GC risk is probably due to the sample size, which might not have been sufficient for the assessment. The differences between our findings and two recent studies can be due to the fact that many lncRNAs display both tumor suppressive and oncogenic functions (24). Human populations are also genetically diverse, and SNPs in lncRNAs largely influence expression (11). In addition, most samples originated from the cardia subsite (63.4%), which are largely different from non-cardia samples based on tumor features, distinct etiologies, and biological behaviors (25). Thus, it can be expected that the mechanisms of lncRNA function in the process of carcinogenesis will be different. Further research involving a larger sample size that encompasses diverse geographic and ethnic groups within Iran is essential to enhance the validity of the findings. This will facilitate a deeper understanding of the functional mechanisms of LINC00659 in GC and elucidate its impact on cellular pathways and tumorigenesis.
![A and B, calculations of fold change (mean ± SE) in expression levels between the normal and tumor paired samples for all samples and for anatomical sites and histopathological types of the tumors. P-values for total, cardia gastric cancer (GC), non-cardia GC, cardia/non-cardia GC, intestinal- and diffuse-type GC [vs. adjacent non-cancerous tissue (ANCTs)] were 0.33, 0.43, 0.36, 0.74, 0.41, and 0.58, respectively. A and B, calculations of fold change (mean ± SE) in expression levels between the normal and tumor paired samples for all samples and for anatomical sites and histopathological types of the tumors. P-values for total, cardia gastric cancer (GC), non-cardia GC, cardia/non-cardia GC, intestinal- and diffuse-type GC [vs. adjacent non-cancerous tissue (ANCTs)] were 0.33, 0.43, 0.36, 0.74, 0.41, and 0.58, respectively.](https://services.brieflands.com/cdn/serve/3170b/73517778cb691c758abf769b34f2a7f8cdf7b28b/jjcmb-16-2-153640-i001-preview.webp)