1. Background
Cancer results from the uncontrolled division of abnormal cells and is a major cause of mortality in economically developed nations. It is also the second most common cause of death in developing countries (1). According to projections from the World Health Organization (WHO), cancer incidence is expected to increase by 69% by 2030, reaching a total of 21 million cases (2). Skin cancer is highly prevalent, with cases rising rapidly. Although deaths from nonmelanoma skin cancer (NMSC) have been declining, the mortality rate for melanoma is unfortunately increasing (3). Phytochemicals have been used as therapeutic agents for treating various diseases for centuries (4). Some phytochemicals, including vinblastine, vincristine, doxorubicin, and topotecan, are recognized for their anticancer properties and have been employed as anticancer drugs with minor modifications (5, 6).
The Ferula genus, part of the Apiaceae family, comprises approximately 170 species primarily distributed across western Asia and northern Africa (7). Various studies suggest that extracts from Ferula species exhibit a wide range of biological effects, including antibacterial, antimicrobial, antioxidant, anti-inflammatory, and anticancer properties (8, 9). In Iranian traditional medicine, Ferula gummosa Boiss, known as Barijeh, is used to treat gastrointestinal disorders and infections (10-12).
Melanoma, a highly lethal and metastatic form of skin cancer (13), has shown increasing incidence in recent years and is now among the top three fatal cancers (14). A-375 cancer cells, which are malignant adherent melanoma cells with epithelial morphology, are widely used as a model for studying carcinogenesis (15).
2. Objectives
Limited research has been conducted on the cellular toxicity and reactive oxygen species (ROS)-neutralizing potential of the gum of F. gummosa in certain tumor cells. However, there is currently no data available on the specific cytotoxic effects of the gum of F. gummosa against A-375 cells. Accordingly, the present study aims to evaluate the cellular toxicity and anticancer properties of the gum of F. gummosa on A-375 cells.
3. Methods
3.1. Preparation of the Gum of Ferula gummosa Gum
In May and June 2022, F. gummosa gum (48590-TARI) was collected from the root source in northeastern Iran. A stock solution of the gum, containing 50 µg/mL dissolved in dimethyl sulfoxide (DMSO), was prepared and stored at -20°C until required.
3.2. Cell Culture
The A-375 and HEK-293T cells were obtained from the National Center for Genetic and Biological Reserves of Iran in Tehran. They were maintained in RPMI-1640 medium (Sigma), supplemented with 10% fetal bovine serum (FBS; Sigma) and 1% penicillin/streptomycin (Sigma).
3.3. MTT Assay
Cell viability was measured using the MTT assay. Briefly, 6 × 10³ cells were plated in each well of a 96-well plate with 150 µL of growth medium. The cells were grown to 70% confluency and subsequently exposed to 2, 5, 10, 20, 30, 40, 50, 60, and 70 µg/µL of the gum for 48 hours.
After discarding the culture medium, 100 µL of PBS containing 5 µg/µL of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) was added to each well. After three hours, the MTT solution was discarded and replaced with 100 µL of DMSO. Absorbance was then measured at 590 nm using a BioTek ELISA reader (USA). Each assay was performed in triplicate.
3.4. Acridine Orange/Ethidium Bromide Staining
A total of 6 × 10³ A-375 cells were cultured on gelatin-coated coverslips in individual wells of a 6-well plate until they reached 70% confluency. The cells were then treated with varying concentrations (4, 6, 8, 10, and 12 μg/mL) of the gum for 48 hours. Subsequently, the cells were washed with PBS and fixed using a 50:50 (v/v) mixture of cooled acetone and methanol for 20 minutes in a freezer.
Following fixation, the coverslip-mounted cells were submerged in a solution containing 100 µg/mL acridine orange (AO) and 100 µg/mL ethidium bromide (EtBr) for 30 minutes. The coverslips were then rinsed with PBS, dried, and observed using ultraviolet microscopy. All experiments were performed in triplicate.
3.5. DNA Laddering
In a 6-well plate, 8 × 10³ A-375 cells were seeded and grown to 70% confluency. Subsequently, the cells were exposed to 4, 6, 8, 9, 10, 11, and 12 μg/mL of F. gummosa gum for 48 hours. The cells were collected using 10% EDTA-Trypsin and centrifuged at 1500 rpm for 5 minutes.
The pellets were treated with a cell lysis buffer containing 0.01 M Tris-HCl (pH 8.0), 0.1 M sodium chloride, 0.025 M EDTA, 1% (w/v) sodium dodecyl sulfate (SDS), and 0.3 mg/mL proteinase K, then incubated at 50°C overnight. The lysate was treated with 10 µg/mL RNase and incubated for 1 hour at 37°C, followed by centrifugation at 13,200 rpm. The upper phase was separated using a solution of phenol, chloroform, and isoamyl alcohol in a 25:24:1 ratio. DNA was then precipitated with ethanol, incubated for 2 hours in a freezer, and centrifuged at 13,200 rpm for 3 minutes.
The resulting pellets were solubilized in 15 µL of dH₂O, loaded onto a 1% agarose gel, and subjected to electrophoresis for 1 hour. The gel was stained with EtBr and visualized using the Red-Type AlphaImager system (Proteinsimple, USA). All experiments were performed in triplicate.
3.6. Clonogenic Assay
The clonogenic assay is a standard method for assessing a single cell's ability to proliferate and generate a colony containing at least 50 cells (16). A total of 50 A-375 cells per well were plated in a 24-well plate in triplicate to allow for adherence. The cells were then exposed to 4, 6, 8, 10, and 12 µg/mL of the gum over a 7-day period.
Following the incubation period, the cells were fixed using a 50:50 (v/v) mixture of cooled acetone and methanol for 15 minutes and subsequently stained with a 0.5% crystal violet solution for 2 hours. The cells were then air-dried, and the colonies were observed using a stereomicroscope (Olympus, Japan). All assays were conducted in triplicate.
3.7. Cell Scratch Assay
Each well of a 6-well plate was seeded with 8 × 10³ A-375 cells and cultured until the cells reached confluence. A scratch was then created in the cell monolayer using a sterile pipette tip, and floating or damaged cells were removed by rinsing with PBS. The cells were subsequently exposed to 4, 6, 8, and 10 µg/mL of the gum for 48 hours.
After treatment, the plates were imaged, and the wound areas were quantified using ImageJ software. All assays were performed in triplicate.
3.8. Statistical Analysis
The results are presented as the mean with standard deviation, derived from three independent experiments. Statistical analysis was performed using SPSS software (IBM, Statistic 25), while IC50 values were calculated using Excel (Office 2016) and GraphPad Prism version 8 (GraphPad Software, La Jolla, CA, USA, http://www.graphpad.com/). Wound surface areas were quantified using ImageJ software version 1.40g (NIH, Bethesda, MD, USA). Results were considered statistically significant when the P-value was less than 0.05.
4. Results
4.1. Effect of the Gum of Ferula gummosa on the Viability of A-375 Cells
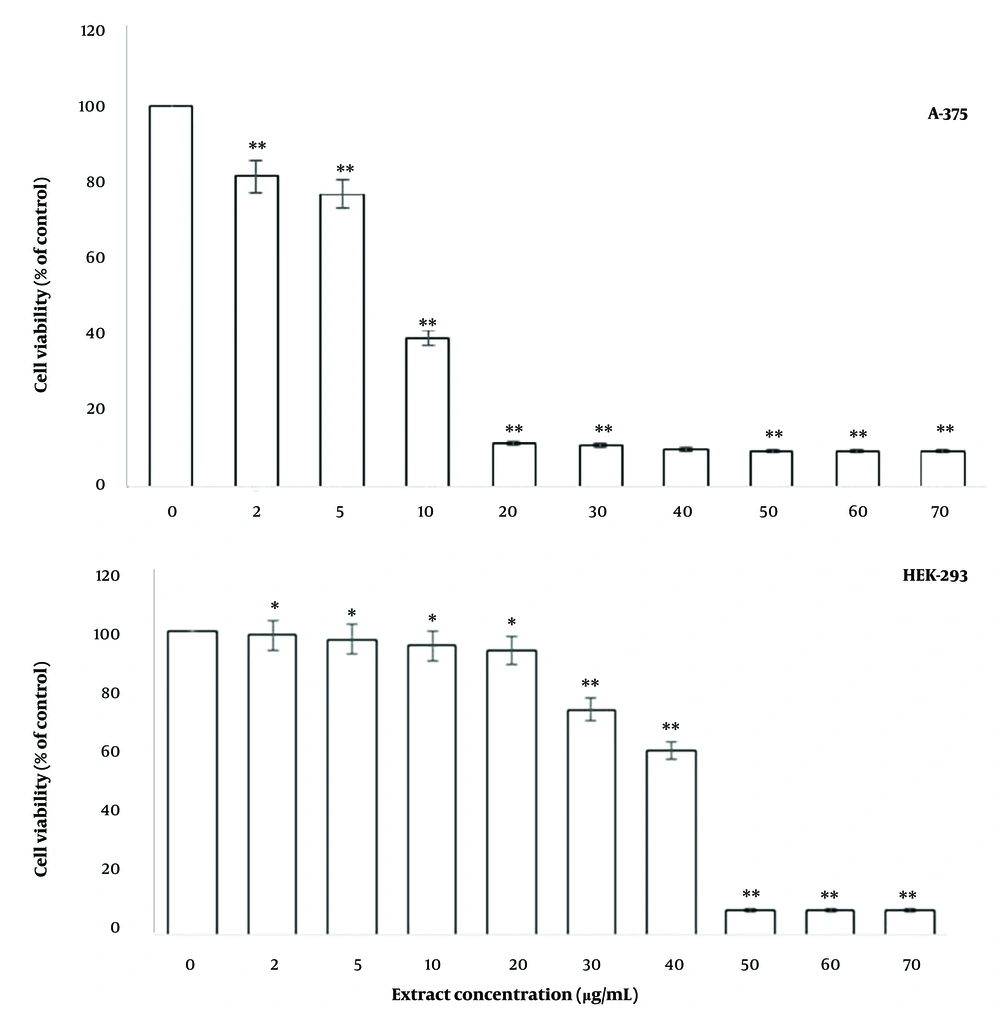
To assess the effect of F. gummosa gum on cell viability, A-375 cells were exposed to varying concentrations of the gum for 48 hours. As shown in Figure 1, exposure to 2 and 5 µg/mL of the gum caused minimal reduction in cell viability. However, concentrations of 10 µg/mL or higher resulted in a significant decrease in cell survival (Figure 1). The IC50 concentration of the gum for A-375 cells was determined to be 8 µg/mL.
The gum of alleviates the survival of A-375 and HEK-293T cells. Cells were plated in every cavity of 96-well plates and grown to 70% confluency. They were subsequently exposed to various doses of the gum for a duration of 48 hr, after which cell survival was assessed by performing the MTT experiment. The results are presented as mean ± SD from three independent experiments, with each treatment conducted in 10 wells (* P < 0.05 and ** P < 0.01 compared to control).
Due to the unavailability of a normal human skin cell line, HEK-293T cells were used as a substitute. It was observed that the gum had a lesser impact on the viability of HEK-293T cells, a type of normal cell, with an IC50 of 38.18 µg/mL (Figure 1).
4.2. Effect of the Gum of Ferula gummosa on the Genomic DNA of A-375 Cells
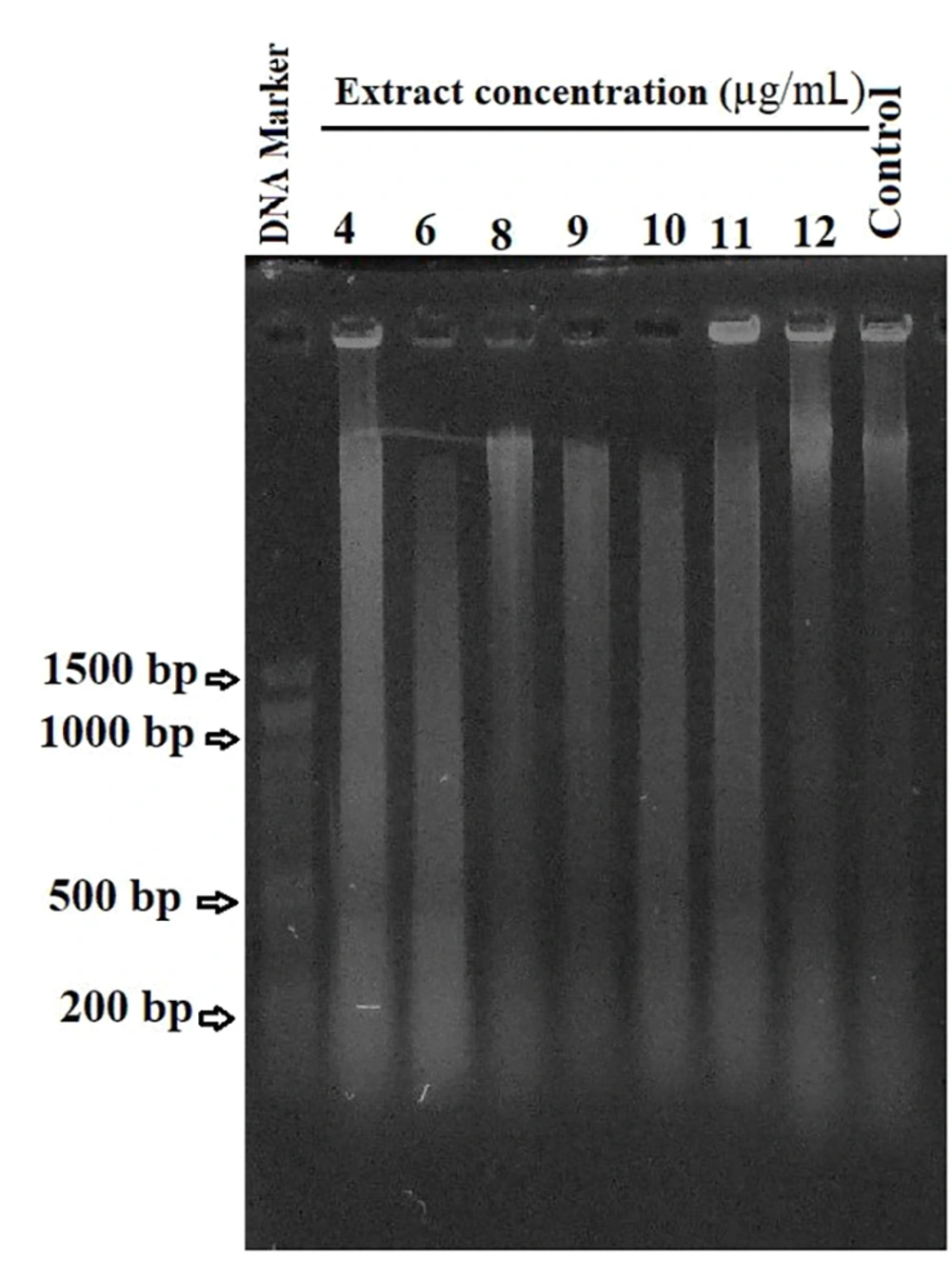
To investigate the potential induction of apoptosis by the gum, a DNA fragmentation experiment was conducted. A-375 cells were treated with varying concentrations (4, 6, 8, 9, 10, 11, and 12 µg/mL) of the gum for 48 hours. After treatment, genomic DNA was extracted from these cells for analysis. Equal quantities of DNA from each sample were separated on a 1% agarose gel and stained with ethidium bromide for electrophoresis.
As shown in Figure 2, a characteristic DNA ladder pattern was consistently absent, while DNA degradation was observed as a smear in the gel. Notably, as the gum concentration increased to 10 µg/mL, the intensity of the smear visibly increased. However, the smear intensity diminished in cells exposed to higher concentrations, such as 11 and 12 µg/mL, indicating a significant reduction in the number of viable cells (Figure 2).
4.3. Impact of Ferula gummosa Gum on the Status of Nuclear Integrity in A-375 Cells
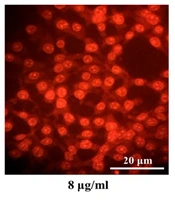
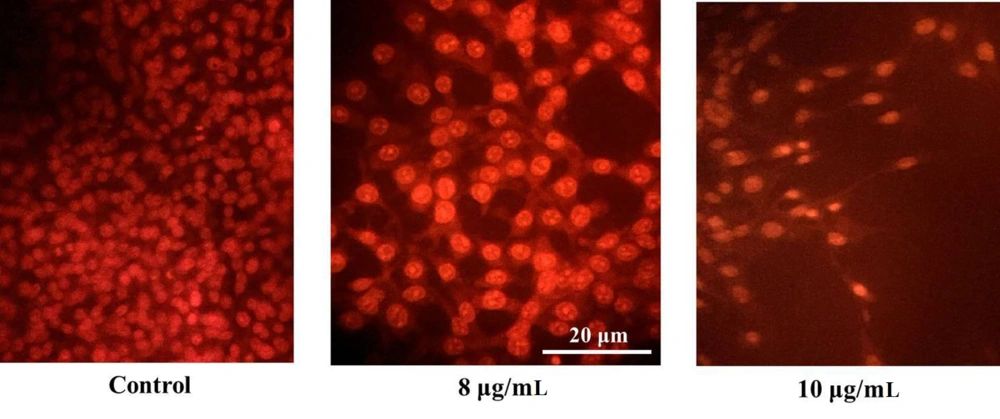
A-375 cells were treated with various concentrations of the gum, stained with AO/EtBr, and observed under a fluorescence microscope. As depicted in Figure 3, no significant nuclear alterations were observed in untreated control cells. However, cells exposed to concentrations near the IC50 value (8 and 10 µg/mL of gum) exhibited morphological changes characteristic of apoptosis, including chromatin compaction, nuclear segmentation, and nuclear margination (Figure 3).
Ferula gummosa gum triggers nuclear condensation in A-375 cells. A-375 cells were plated in 6-well plates and subjected to various doses of the gum for 48 hr. A-375 cells exposed to 8 and 10 µg/mL concentrations of the gum exhibited nuclear compaction and fragmentation. In contrast, these alterations were not seen in the control (untreated) cells.
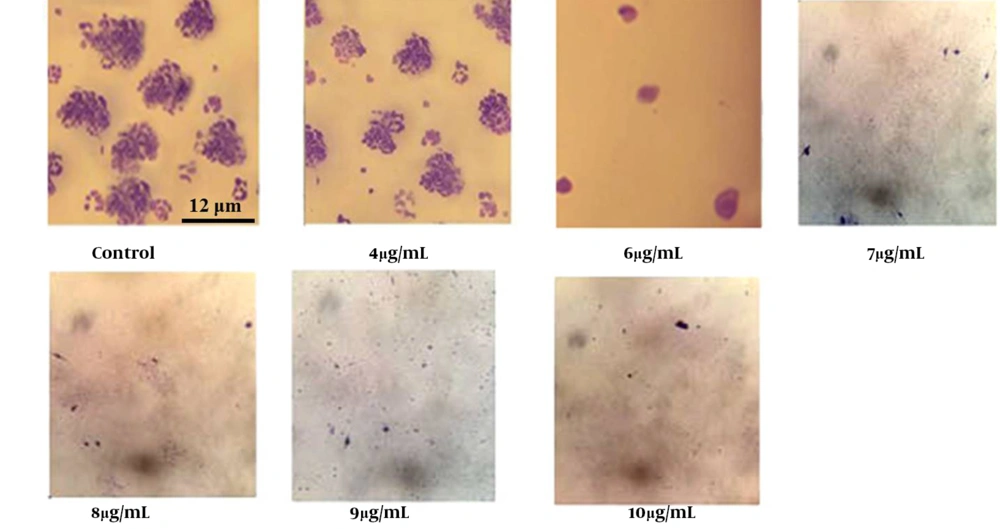
4.4. Impact of Gum Treatment on Colony Formation of A-375 Cells
A colony formation assay was performed to evaluate the inhibitory effects of the gum on the viability of A-375 cells. As shown in Figure 4, the colony-forming ability of cells treated with 4, 6, 7, 8, 9, and 10 µg/mL of gum decreased in a dose-dependent manner. Notably, cells treated with concentrations above the IC50 completely failed to form colonies.
The plating efficiency (PE) for untreated control cells was 60%, while the survival fraction (SF) at gum concentrations of 8, 9, 10, and 12 µg/mL was calculated to be 1.7, 1.2, 0.95, and 0.58, respectively (P < 0.01) (Figure 4).
The gum reduces the colony-forming capacity of A-375 cells in a concentration-dependent way. A-375 cells were plated into a 24-well plate, and let to adhere to the surface. After adhesion they were exposed to various doses of the gum for a duration of 7 days. After fixation and staining were completed, the colonies were analyzed.
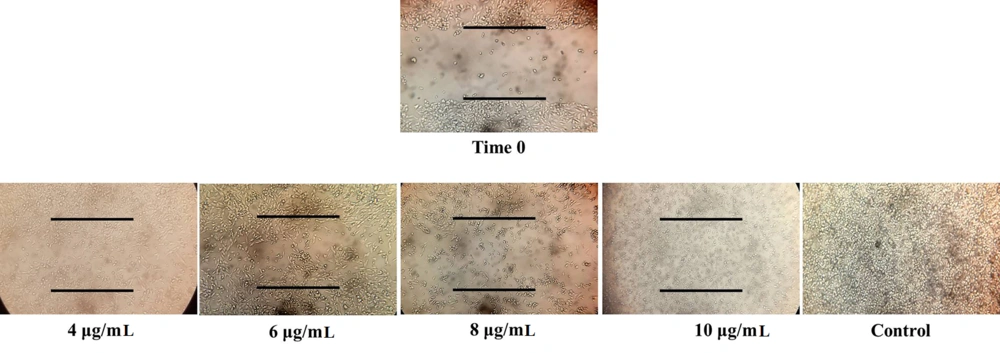
4.5. Impact of the Gum on the Invasion of A-375 Cells
An in-vitro scratch (wound-repair) assay was conducted to evaluate the effect of F. gummosa gum on the invasion ability of A-375 cells. As illustrated in Figure 5, the gum significantly reduced cellular growth and migration in a dose-dependent manner compared to the control group. At a concentration of 10 µg/mL, the gum exhibited cytotoxic effects, causing cell detachment and floating in the well.
5. Discussion
This study aimed to investigate the cytotoxic and anti-cancer properties of F. gummosa gum, a medicinal plant native to specific regions of Iran. Previous research by our team has revealed that the gum contains diverse chemical compounds and exhibits significant antioxidant potential in PC-3 and SW-480 cells (17, 18).
In this study, F. gummosa gum demonstrated notable cytotoxic activity against A-375 cells, with an IC50 of 8 µg/mL, while exhibiting much lower cytotoxicity against HEK-293 cells, with an IC50 of 38.18 µg/mL (Figure 1). A review of existing literature suggests that the inhibitory effects of the gum on A-375 cells are comparable to those observed with doxorubicin and cisplatin (19, 20).
DNA degradation experiments indicated that the reduction in cell survival in A-375 cells was associated with DNA disintegration and induction of cell death. Remarkably, DNA fragmentation was detected across all concentrations of F. gummosa gum tested (Figure 2).
Acridine orange/ethidium bromide (AO/EtBr) staining results further confirmed that treatment with gum concentrations of 8 µg/mL and above led to chromatin condensation, nuclear fragmentation, and nuclear margination—key morphological features characteristic of apoptosis (Figure 3).
The clonogenic assay results demonstrated that F. gummosa gum inhibits the proliferation of A-375 cells in a concentration-dependent manner, as shown in Figure 4. Additionally, our previous research underscored the gum's significant inhibitory effects on SW-480 cells (18).
According to the scientific literature, cellular multiplication and invasion are essential processes in wound-repair mechanisms (21-23). Our findings indicated that exposure to non-lethal doses (2 and 5 µg/mL) of the gum did not significantly affect the survival of A-375 cells. However, when A-375 cells were exposed to toxic concentrations of the gum, a marked reduction in the rate of wound repair in the cell layers was observed. This suggests that the decline in wound recovery in monolayer cultures exposed to the gum is likely due to diminished invasive behavior (Figure 5).
Phytochemical analysis of F. gummosa gum identified a high concentration of terpenes, including Bulnesol, β-Pinene, β-Terpinene, δ-Cadinene, β-Thujene, β-Phellandrene, and Valencene (18). These compounds may play a role in modulating the wound-healing process. Further research is necessary to confirm this hypothesis and to determine whether one or more of these compounds are responsible for the gum's inhibitory effects on the invasive behavior of A-375 cells.
5.1. Conclusions
In summary, this preliminary study investigates the cytotoxic and anti-cancer properties of F.gummosa gum on A-375 cancer cells. The findings, corroborated by prior research, indicate that F. gummosa gum possesses significant antioxidant and cytotoxic effects, positioning it as a promising candidate for further exploration. Comprehensive analysis of the gum's chemical composition will be crucial in evaluating its full anti-cancer potential and elucidating the molecular mechanisms underlying its effects.