1. Background
Post-traumatic stress disorder (PTSD) and depression are stress-induced mood disorders that frequently co-occur (1-3). Various studies have demonstrated that anxiety and depression disorders are associated with abnormalities in the structure and function of corticolimbic areas involved in the stress response, including the hippocampus (4-11). The hippocampus, due to its plastic and sensitive nature throughout life, is particularly prone to alterations in response to different stimuli (12). Magnetic resonance imaging brain volumetry has shown reduced hippocampal volume in patients with psychiatric disorders (4, 5, 9, 10). At the synaptic level, studies have revealed that stress-induced disorders alter hippocampal plasticity (8, 13). Furthermore, a growing body of evidence indicates that stress-induced deficits in synaptic plasticity are linked to alterations in autophagy and cytoskeletal elements (14-21).
Autophagy is a lysosomal degradation pathway that removes damaged cellular proteins and organelles (22). Transcription factors play a significant role in regulating the autophagic process (23). One of the key regulators of gene expression in autophagy is transcription factor EB (TFEB) (24). The TFEB is critically involved in the biosynthesis and function of lysosomes and mitochondria (25). Additionally, the formation of autophagosomes, their fusion with lysosomes, and cargo identification in the autophagy process are TFEB-dependent (25, 26). Studies have shown that TFEB-mediated autophagy contributes to synaptic plasticity (27). It is notable that many neurodegenerative diseases result from abnormal protein accumulation (28), reflecting neuronal autophagy dysfunction. Changes in TFEB activity or the expression of its target genes play a crucial role in neuronal diseases (29).
Microtubules and their associated proteins play a crucial role in the development and maintenance of synapses (30, 31). Stathmin, a regulatory protein of microtubule dynamics, is essential for neural dendritic growth and synapse formation (32). Additionally, stathmin regulates the synaptic localization of the GluA2 subunit of AMPA receptors (33). The dependency of hippocampal plasticity on stathmin-mediated microtubule dynamics has also been demonstrated (33). On the other hand, evidence indicates that stathmin expression and activity are altered in mood disorders (33-36).
There is a significant overlap between anxiety and depression regarding their pathogenesis and symptoms, suggesting that these conditions may share common biological mechanisms. However, important distinctions exist between the disorders. For instance, large-scale neural circuit dysfunctions differ between depression and anxiety (37, 38). Furthermore, differences in theta oscillations (39) and emotional states (40) have been identified in patients with depression and anxiety.
Given the critical role of the hippocampus in the pathogenesis of mental illnesses (41), identifying disease-specific molecular changes in the hippocampus and understanding the aspects of hippocampal plasticity disrupted in anxiety or depression are essential for developing effective treatments.
2. Objectives
In the present study, we aimed to compare the levels of TFEB and stathmin proteins in animal models of PTSD and depression.
Post-traumatic stress disorder is a psychological disorder that occurs in some individuals after experiencing or witnessing stressful events. The main symptoms of PTSD include flashbacks, anxiety, negative thoughts, and nightmares, which are associated with various physiological effects (42, 43). The PTSD and depression-like disorders were induced using well-established and widely used rat models, including the single-prolonged stress (SPS) model for PTSD and the maternal separation (MS) model for depression (44-49).
3. Methods
3.1. Animals
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 80–23, revised 1996) and were approved by the Research Committee of Tehran Medical Sciences, Islamic Azad University. Adult male and female Wistar rats were obtained from the Pasteur Institute, Tehran, Iran, and mated in a controlled laboratory environment. The room conditions included a 12-hour light/dark cycle, a temperature of 22 ± 1°C, and free access to water and food. All experiments were carried out between 10:00 a.m. and 2:00 p.m., and each experimental group comprised 6 to 8 animals.
3.2. Experimental Design
The male rat pups were randomly assigned to one of three experimental groups (n = 8). The first group was the depressed group, exposed to maternal separation stress and other related stresses to create a depressed animal model. The second group was the PTSD group, exposed to single-prolonged stress. The remaining group served as the control group.
3.3. Depression Animal Model
To induce depression, the maternal separation procedure was performed on male rat pups. For this purpose, on the 10th day after birth, male pups were subjected to daily separations (30 or 60 minutes) from their mother. The animals were also exposed to hitting, shaking, tilting the cage, and placing them in a moistened cage. This stress procedure was continued for three weeks (50, 51).
3.4. Post-traumatic Stress Disorder Animal Model
The procedure for inducing PTSD was SPS. The protocol involved multiple consecutive stressors, including a 2-hour restraint stress followed immediately by a 15-minute forced swim stress in an acrylic cylinder (20 cm in diameter, filled two-thirds with water at a temperature of 24°C). Afterward, the animals were allowed to recuperate for 15 minutes before being exposed to isoflurane inhalation until loss of consciousness (52).
3.5. Behavioral Tests
Behavioral experiments included the elevated plus maze (EPM) and forced swimming test (FST), conducted with control, PTSD, or depressed rats as follows:
- Experiment 1 evaluated anxiety-like behaviors using the EPM test in PTSD or depressed animals.
- Experiment 2 evaluated depressive-like behaviors using the FST task in PTSD or depressed animals.
3.6. Elevated Plus Maze
The EPM test is a widely used method to measure anxiety-related behavior. The EPM apparatus, constructed from plexiglass, consists of two opposite open arms and two opposite closed arms (50 × 10 cm) arranged in a plus shape and elevated 50 cm above the floor. The animal is placed in the center of the platform, facing one of the open arms, and allowed to move freely for a 5-minute period. Animal behavior is monitored using EthoVision software (version 7.1). The percentage of open arm entries [OAE, calculated as (entries into open arms/total entries) × 100] and the percentage of open arm time [OAT, calculated as (time spent in open arms/total time spent in any arms) × 100] are used to estimate anxiety-like behavior. Total arm entries are recorded as an index of locomotor activity (53).
3.7. Forced Swimming Test
The FST was conducted following the method previously described (54, 55). The test consists of two sessions: A 15-minute pretest habituation forced swim session followed by a 5-minute test session conducted 24 hours later. Each rat was individually placed in a water-filled glass cylinder (height 45 cm, diameter 19 cm) containing 28 cm of water maintained at 23 - 24°C. During the test session, the duration of immobility and swimming time was recorded.
3.8. Brain Tissue Collection
At the end of the experimental sessions, the rats were deeply anesthetized with a ketamine (120 mg/kg) and xylazine (40 mg/kg) mixture. Subsequently, the animals were euthanized using carbon dioxide. To evaluate TFEB and stathmin protein expression, the hippocampi (n = 5) were dissected, immediately frozen in liquid nitrogen, and then stored at -80°C.
3.9. Western Blotting
The extracted hippocampal tissues were homogenized in a lysis buffer and then centrifuged. Protein samples were separated by electrophoresis on a 12% SDS polyacrylamide gel and transferred onto a PVDF membrane. The membranes were fixed and blocked using TBST for 60 minutes at room temperature. They were then incubated overnight at 4°C with primary antibodies (Cell Signaling Co., USA). After three washes with TBST, the membranes were incubated with secondary antibodies (Cell Signaling Co., USA) for 60 minutes at room temperature. Following another three washes with TBST, the membranes were treated with ECL Plus reagent for 1 - 30 minutes. ß-actin was used as an internal control for protein loading, ensuring that all measurements were standardized against ß-actin values.
3.10. Statistical Analysis
The results were analyzed using Prism software (v). One-way ANOVA and post hoc Tukey tests were conducted to evaluate statistical differences between groups in behavioral tasks. Additionally, statistical differences between groups in protein levels were assessed using an independent t-test. Results are expressed as mean ± standard error of the mean (SEM.).
4. Results
4.1. Post-traumatic Stress Disorder Rats Displayed Significantly Higher Anxiety Parameters in Elevated Plus Maze Compared to the Depressed Group Rats
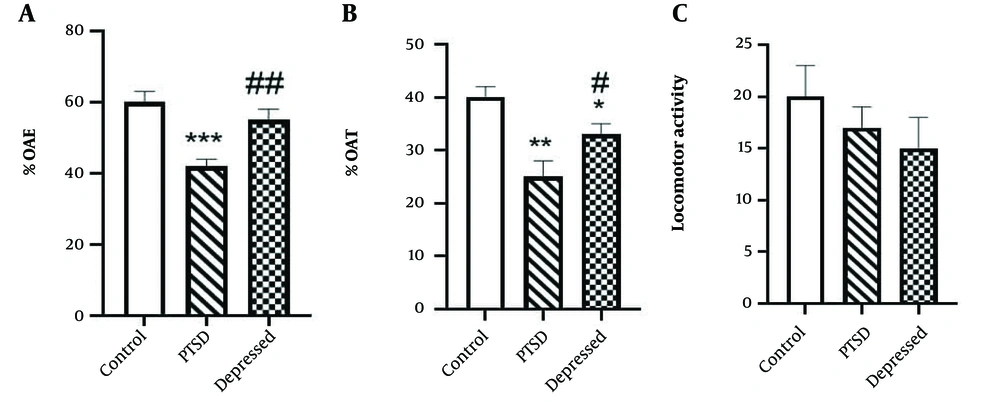
Figure 1 illustrates anxiety-like behaviors in PTSD or depressed animals using the EPM test. Our results revealed that PTSD rats exhibited anxiety-like behaviors in the EPM test compared to control animals. Specifically, the %OAT and %OAE (P < 0.001) decreased in PTSD rats, indicating a PTSD-induced anxiogenic effect (Figure 1A and B). In the depression paradigm, there was a decrease in the percentage of time spent in the open arms (%OAT) (P < 0.05), while the %OAE was not significantly affected compared to the control group (P > 0.05). It is important to note that PTSD or depression-induced behavioral changes occurred without any alterations in locomotor activity (Figure 1C).
Anxiety-like behaviours in the post-traumatic stress disorder (PTSD) or depressed animals using elevated plus maze (EPM) test. A, percentage of time spent in open arms (%OAT); B, percentage of entries into the open arms (%OAE); and C, locomotor activity were measured during 5 min. Data are expressed as the means ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. control group; ## P < 0.001 and # P < 0.05 vs. PTSD group.
4.2. Exposure to the Forced Swimming Test Resulted an Increase in Depressive Behaviours in Post-traumatic Stress Disorder and Depressed Rats
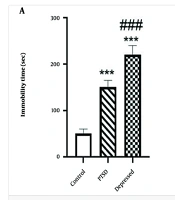
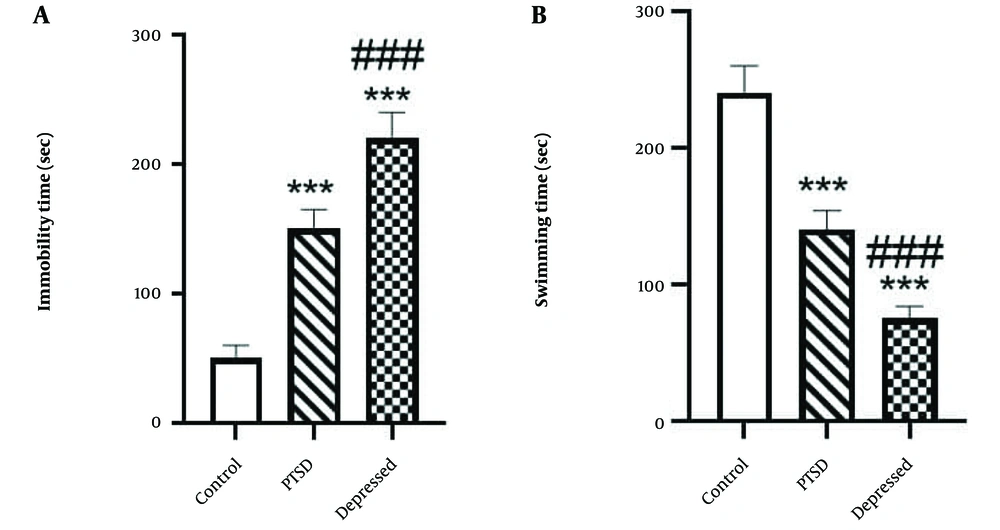
The FST findings demonstrated that in PTSD rats, swimming time was significantly reduced (Figure 2A P < 0.001, effect size: 41.67%), while immobility time was significantly increased (Figure 2B P < 0.001, effect size: 3) compared to the control group. Depression induced a similar effect on immobility time (Figure 2A P < 0.001, effect size: 4.4) and swimming time (Figure 2B P < 0.001, effect size: 68.75%) in the FST compared to the control group (Figure 2). Furthermore, there were significant differences between the PTSD and depressed rats in the FST paradigm (P < 0.001).
Depressive –like behavior of post-traumatic stress disorder (PTSD) or depressed animals using forced swimming test (FST) test. A, immobility time; and B, swimming time were measured during 5 min. Data are expressed as the means ± SEM. *** P < 0.001 compared with control group. ### P < 0.001 vs. PTSD group.
4.3. The Level of Transcription Factor EB and Stathmin Proteins Was Changed in the Hippocampus of Post-traumatic Stress Disorder and Depressed Rats
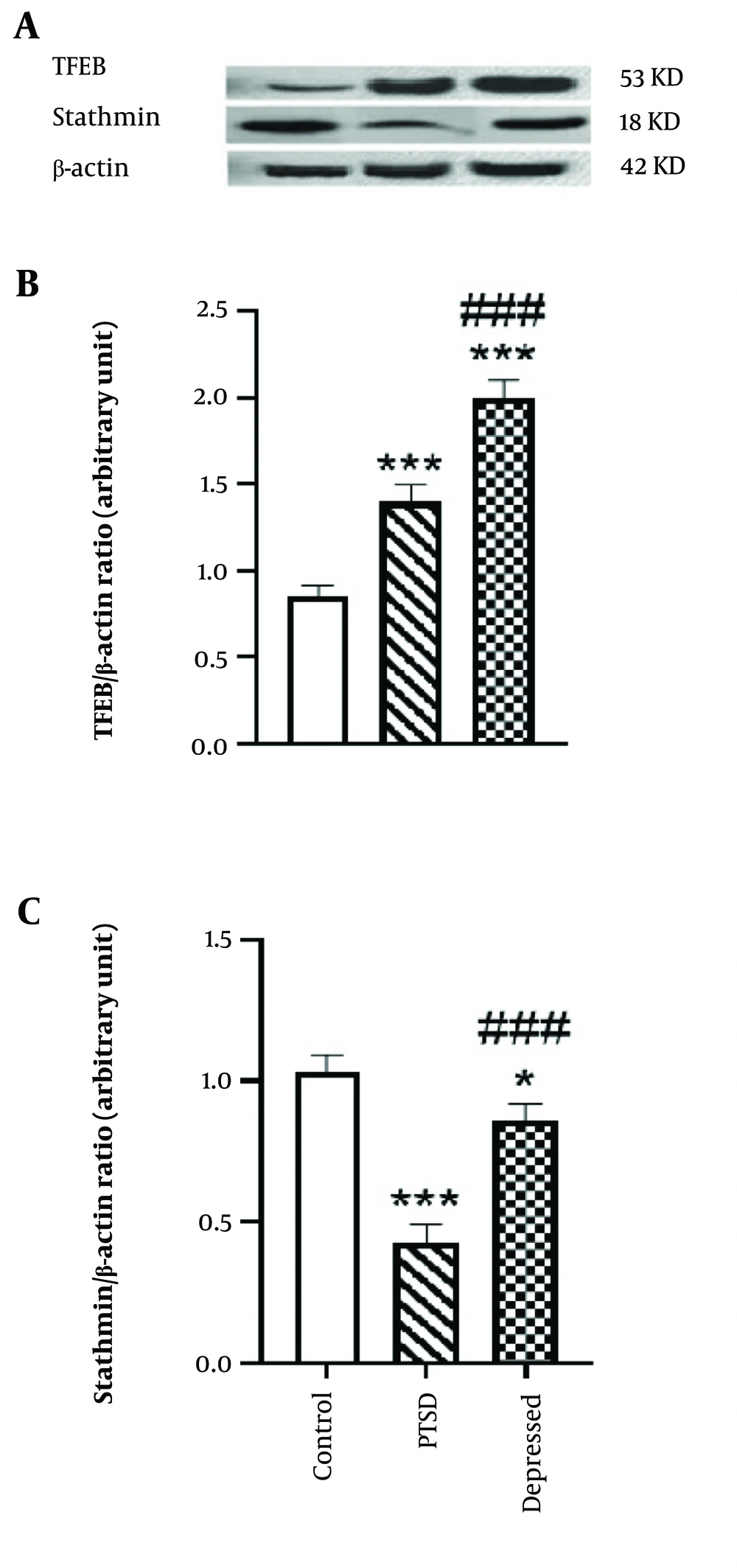
Figure 3A shows that the hippocampal level of TFEB was significantly increased in PTSD (P < 0.001, effect size: 1.87) and depression models in rats (P < 0.001, effect size: 2.5) compared to the control group (Figure 3B). Additionally, the level of stathmin protein was significantly decreased in PTSD (P < 0.001, effect size: 20%) and depression models in rats (P < 0.001, effect size: 60%) compared to the control group (Figure 3C). A comparison of stathmin protein levels between PTSD and depressed rats also revealed a statistically significant reduction in stathmin levels in the PTSD group relative to the depressed group (P < 0.001). These results indicate that PTSD and depressive disorders are associated with increased TFEB levels and decreased stathmin protein levels in the hippocampus.
A, hippocampal level of transcription factor EB (TFEB) and stathmin in post-traumatic stress disorder (PTSD) and depressed rats. B and C, bars represent fold differences of mean normalized expression value ± SEM. * P < 0.05 vs. control group, *** P < 0.001 compared with control group. ### P < 0.001 compared with PTSD group.
5. Discussion
To date, various animal models have been developed to understand the molecular mechanisms involved in the pathogenesis of depression and PTSD (27, 48). Daily repeated MS is one of the most commonly used experimental procedures in depression studies (45, 47). Both human and animal studies have shown that MS increases the tendency toward depression- and anxiety-like behaviors in adulthood (7, 56-60). Consistent with these findings, our study demonstrated that postnatal maternal separation induced anxiety- and depressive-like behaviors in adult animals. Specifically, we found that rats subjected to maternal separation stress spent less time in the open arms of the EPM task compared to the control group, without any changes in locomotor activity, reflecting anxiety-like behavior. Additionally, in the FST, maternal separation increased immobility time while reducing swimming time compared to the control group, correlating with depressive-like behaviors.
In PTSD animals, we observed elevated levels of anxiety and depression in both EPM and FST tasks compared to the control group, aligning with previous studies (44, 46, 48, 49). The behavioral results from our study support the co-occurrence of anxiety and depression under stress conditions (61). Notably, PTSD rats exhibited greater anxiety behavior on the EPM compared to the depressed group, while both groups displayed similar behaviors in the FST.
It is well-established that under anxious or depressive conditions, autophagic mechanisms are impaired in the hippocampus (8, 62, 63). This impairment may stem from the parallel roles of the hippocampus and autophagy in the stress response. Autophagy serves as a major stress response in the central nervous system (64), and the hippocampus is central to processing stress responses (as reviewed in the introduction). Interestingly, pharmacotherapies that reduce depression-like symptoms often affect the autophagy-lysosomal pathway (65).
TFEB is a transcriptional regulator of autophagy, and upon activation, it enhances autophagosome formation, lysosome function, and autophagic flux (66). Recently, TFEB overexpression has been proposed as a therapeutic intervention for neurodegenerative diseases (15, 67, 68). Our previous study revealed that TFEB signaling in the amygdala and medial prefrontal cortex (PFC) plays a pivotal role in processing anxiety and depression (69). However, to our knowledge, changes in hippocampal TFEB protein levels in PTSD or depression have not been studied.
Our results showed that PTSD or depressed rats exhibited an increase in the hippocampal level of TFEB. These findings align with the study by Wan et al., which reported an increase in hippocampal autophagosomes in SPS-exposed rats (70). However, they contrast with our previous study that observed decreased TFEB mRNA levels in the amygdala or PFC regions (69). Previous studies have also demonstrated a decrease in hippocampal mammalian target of rapamycin (mTOR), an inhibitor of TFEB, in maternally separated mice and in major depressive disorder (71). When mTOR is inhibited, TFEB is activated and translocates to the nucleus, triggering the expression of its target genes (68). Interestingly, TFEB can increase its own expression by binding to its promoter (26).
In contrast, Zhang et al. reported an increase in mTOR levels in the hippocampus of stressed mice in the chronic restraint stress model of depression (72). Furthermore, Liu et al. showed region-dependent changes in autophagic marker expression in MS-treated animals (7). These findings suggest that the TFEB response during anxiety or depression is complex. Given that TFEB expression correlates with autophagy enhancement, we propose that the upregulation of autophagy following TFEB expression in this study contributed to the observed anxiety and depression behaviors.
There is considerable evidence demonstrating that alterations in synaptic plasticity mediated by the cytoskeletal microtubular system play a crucial role in the pathogenesis of PTSD and depression (35, 73, 74). Stathmin, a negative regulator of microtubule stability, is present in dendritic spines (75, 76) and modulates synaptic plasticity by regulating the dendritic localization of the GluA2 subunit of AMPA-type glutamate receptors (AMPARs) (31). Moreover, mice lacking stathmin display impaired long-term potentiation (LTP) in their cortico-amygdala and thalamo-amygdala pathways and exhibit deficits in learned conditioning (76). Several studies support the role of stathmin in anxiety, social behavior, depression, and fear (34, 77, 78).
In the present study, our data revealed a reduction in hippocampal stathmin protein levels in both PTSD and depressed rats compared to the control group. This finding is consistent with the study by Han et al., which showed that SPS or immobilization stress-induced a reduction in stathmin expression in the hippocampal area (79).
We also compared hippocampal TFEB and stathmin protein expression levels between PTSD and depressed rats. The results revealed differences in their expression levels. The reduction of stathmin in the PTSD group was greater than in the depressed animals, while the enhancement of TFEB expression in depressed rats was significantly higher than in PTSD rats.
5.1. Conclusions
In summary, the findings of this study suggest that autophagy is upregulated in both anxiety and depression. However, based on the differing levels of TFEB in PTSD and depressed rats, we conclude that depression is more closely linked with autophagy dysfunction. Conversely, considering the expression of stathmin, anxiety appears to be more associated with microtubule alterations. Despite the overlapping features between anxiety and depression, these results indicate that there are anxiety-specific and depression-specific neuroplasticity changes that should be taken into account for future treatment strategies.