1. Background
Improving sports performance is of paramount importance. Coaches and athletes focus on physiological factors such as strength, VO₂max, and anaerobic capacity to achieve success (1). Weightlifters, in particular, require these physiological adaptations to lift heavy weights effectively. These athletes demonstrate exceptional explosive power compared to others (2, 3), which is a critical component for optimal performance (3). The positive impact of weight training on anaerobic capacity and the cardiovascular system has been well established (4). However, the search for innovative methods to enhance power and reduce fatigue in weightlifters continues.
Research indicates that weightlifting exercises improve jumping ability and speed performance (5). However, there remains a gap in understanding how to further enhance performance and power in weightlifting. Blood flow restriction (BFR) training has been shown to improve anaerobic power, but it is often limited by movement restrictions, pain, and athletes' intolerance at higher intensities (6).
An alternative training method used in endurance exercises involves the use of an added respiratory dead space (ARDS) device. This device, which includes an additional corrugated tube of a specified length without a valve to increase respiratory resistance, has shown potential for improving buffering capacity (7, 8). Studies on the ARDS method have reported increases in partial pressure of carbon dioxide (PCO₂) and decreases in blood pH (9). These physiological changes, along with increased bicarbonate (HCO₃⁻) concentrations, may lead to delayed acidosis, improved buffering capacity, and enhanced energy generation through anaerobic metabolism (10).
In mammalian cells, oxygen homeostasis is regulated by the transcription factor hypoxia-inducible factor 1-alpha (HIF-1α). Hypoxia-inducible factor 1-alpha regulates the expression of genes that enable cell adaptation to low oxygen tension, including genes involved in angiogenesis, glucose metabolism, and glucose transport. Research highlights a significant relationship between anaerobic power and HIF-1α in athletes (11-13). Notably, scientists have observed that athletes focused on power and explosive performance possess a unique form of HIF-1α, which enhances their performance (11, 12). Additionally, lactate (La⁻), a byproduct of glycolysis and a key signaling molecule, has been shown to prevent the degradation of HIF-1α (13).
However, to the best of our knowledge, the ARDS training method has only been studied in the context of aerobic exercises. Studies by Zaton et al. and Smolka et al. reported improved exercise performance following endurance training interventions using the ARDS method (9, 14). Similarly, in the study by Danek et al., increased performance was observed in sprint interval exercises (SIE) with varying volumes of ARDS, without significant changes in the rating of perceived exertion (RPE) or fatigue (15).
Overall, since the ARDS training method facilitates the development of anaerobic metabolism, the present study aimed to investigate whether applying this method to weightlifting exercises could capitalize on the increase in lactate (La⁻) to enhance HIF-1α and ultimately improve anaerobic power.
2. Objectives
Therefore, this study examined the effects of increased respiratory CO₂ during exercise on buffering capacity, HIF-1α levels, anaerobic power, Fatigue Index (FI), and RPE over the course of 10 weeks of weightlifting training with added dead space.
3. Methods
Among 30 male weightlifters with a maximum of six months of weightlifting training experience (mean age: 28.2 ± 3.02 years), 20 men were selected through purposive convenience sampling. The sample size was determined using G*Power software and data from previous studies, identifying a range of 18 - 20 participants. The health status of the participants was assessed through a self-report form and the Physical Activity Readiness Questionnaire (PAR-Q). They were then randomly assigned, based on their body mass index (BMI between 20 and 25), into two groups: weightlifting with a mask (WARDS = 10 participants) and weightlifting without a mask (WT = 10 participants).
Inclusion criteria were no history of cardiovascular disease, diabetes, hypertension, respiratory diseases, non-smoking, and not following any diet or weight gain/loss programs. Exclusion criteria included taking any medication without prior notice, skeletal and respiratory diseases, and missing more than one-third of the training sessions. Written consent forms were obtained from all participants. Ultimately, data from 18 participants were evaluated (Table 1).
| Variables and Groups | Pre-test | Post-test | Percentage of Change | P | ||
|---|---|---|---|---|---|---|
| Intergroup | Between-Group | F | ||||
| Age (y) | ||||||
| WARDS | 28.3 ± 2.17 | - | - | - | - | - |
| WT | 28.2 ± 3.83 | - | - | - | - | - |
| Body height (cm) | ||||||
| WARDS | 174.5 ± 9.47 | - | - | - | - | - |
| WT | 179.7 ± 7.07 | - | - | - | - | - |
| Body mass (kg) | 0.714 | 0.457 | ||||
| WARDS | 74.9 ± 8.86 | 76.4 ± 9.63 | 2.01 | 0.983 | ||
| WT | 77.8 ± 8.47 | 79.5 ± 8.39 | 2.18 | 0.977 | ||
| BMI (kg/m2) | 0.510 | 0.787 | ||||
| WARDS | 24.5 ± 1.59 | 25.0 ± 1.62 | 1.95 | 0.883 | ||
| WT | 24.0 ± 1.07 | 24.5 ± 1.08 | 2.20 | 0.844 | ||
| BFP (%) | 0.942 | 0.130 | ||||
| WARDS | 13.7 ± 1.11 | 13.4 ± 0.98 | -2.46 | 0.929 | ||
| WT | 13.5 ± 1.44 | 13.5 ± 1.22 | -0.29 | 1.000 | ||
General Characteristics of the Subjects a
3.1. Technical Information
On the first day of the participants’ arrival at the laboratory, measurements of height (using a Seca stadiometer, Germany), weight, and body composition (using an InBody 770 analyzer, South Korea) were conducted. These measurements were taken 48 hours after the protocol and under similar environmental conditions.
3.1.1. Anaerobic Power and Fatigue Index
The 30-second Wingate test was performed on a cycle ergometer (Monarch, model 894) with a load equivalent to 7.5% of the participant’s body mass. All tests were conducted in the early morning following a light breakfast. Participants were instructed to start pedaling 10 seconds before the workload was applied and to continue with maximum effort for the duration of the 30-second test. Variables including peak anaerobic power and FI obtained from the test were recorded.
3.1.2. Rate of Perceived Exertion
The Borg scale (10-point) was used to measure RPE. Participants were verbally asked to report their perceived exertion after completing each exercise set, and the average RPE was calculated for that training day.
3.1.3. One Repetition Maximum
Participants selected the weight based on their initial estimate and performed the movement until failure. The amount of weight lifted and the number of repetitions completed were used in the relevant formula to calculate the 1RM:
3.2. Laboratory Method
Blood samples were collected to measure lactate (La-), partial pressure of carbon dioxide (PCO₂), and bicarbonate (HCO₃⁻) levels at six stages: Twenty-four hours and one hour before the start of the first training session, one hour before the start of the last training session, immediately after the first and last training sessions, and 48 hours after the last training session. Additionally, a diagnostic kit was used to measure La- and HIF-1α levels at two stages: Before and after the training protocol.
3.3. Training Protocol
Participants underwent weightlifting training under the supervision of an experienced international coach for two weeks, attending three sessions per week to address and correct technical flaws. Following this preparatory phase, all participants engaged in the main weightlifting training program for eight weeks. Each session included two out of four primary weightlifting movements: Power snatch, squat snatch, power clean and push jerk, and squat clean and split jerk. The training sessions progressively increased in frequency and intensity (Table 2). The primary distinction between the two groups was that the WARDS group used masks and tubes throughout the training sessions, except during the warm-up, cool-down, and a 10-minute rest period between exercises.
| Weeks | Activity | Frequency | Rep × Set (for Each Movements) | Intensity (RPE) | Intensity (%) 1RM |
|---|---|---|---|---|---|
| 1 - 2 | Coordination-practice | 3 | 5 × 6 - 8 | 14 - 16 | 80 |
| 3 - 4 | Main exercise | 3 | 5 × 6 - 8 | 14 - 16 | 80 |
| 5 - 6 | Main exercise | 4 | 5 × 6 - 8 | 14 - 16 | 80 |
| 7 - 10 | Main exercise | 5 | 5 × 6 - 8 | 14 - 16 | 80 |
Weight Lifting Training Method a
3.3.1. Dead Space Increasing Device
The device used consisted of an oxygen mask connected to a ventilator tube measuring 402 cm in length and 2.5 cm in diameter, creating 1000 mL of dead space (15).
3.4. Statistical Method
The test results were analyzed statistically by calculating the mean and standard deviation values. Normality and homogeneity of variance were assessed using the Shapiro-Wilk and Levene’s tests. To examine the main effects and interactions between the group factor (WT vs. WARDS) and time factor (pre-training vs. post-training), a repeated measures ANOVA with a between-group factor was applied. If significant differences between groups were identified, Bonferroni post hoc tests were used for further analysis. All statistical calculations were conducted using SPSS software version 22, and charts were created in Excel. A significance level of P ≤ 0.05 was considered for all tests.
4. Results
The results regarding the general characteristics of the subjects are presented in Table 1. The findings indicate that after 10 weeks of training, the indices for weight (P = 0.714), BMI (P = 0.514), and body fat percentage (BFP, P = 0.942) did not exhibit significant between-group changes.
Table 3 presents the results related to blood gases and La- across six stages. The findings reveal that after 10 weeks of training, there was a significant inter-group change in the variables PCO2 (P = 0.031), HCO3- (P = 0.029), and La- (P ≤ 0.001). The intra-group analysis showed a significant difference in the variable PCO2 in the WARDS group (after the first and last session) and in the WT group (after the first session). Additionally, significant differences were observed in the variables HCO3- and La- in the WARDS group (after the first and last session).
| Variables and Groups | 24 Hours Before Training | Before the First Session | Immediately After the First Session | Before the Last Session | Immediately After the Last Session | 48 Hours After the Last Session | P-Value b |
|---|---|---|---|---|---|---|---|
| PCO2 (mmHg) | 0.031 | ||||||
| WARDS | 51.1 ± 3.6 | 52.6 ± 5.78 | 71 ± 11.5 c | 56.6 ± 5.8 | 92.3 ± 13.8 c | 51.3 ± 4.1 | |
| WT | 51.3 ± 4.9 | 52.6 ± 7.11 | 56.3 ± 7.18 c | 52.3 ± 6.9 | 72.9 ± 17.5 | 51.2 ± 4.9 | |
| HCO3- (mg/L) | 0.029 | ||||||
| WARDS | 30.0 ± 3.54 | 31.3 ± 2.07 | 35.8 ± 2.70 c | 34.2 ± 2.47 | 38.6 ± 2.68 c | 30.6 ± 2.56 | |
| WT | 30.2 ± 2.53 | 30.8 ± 1.96 | 31.6 ± 0.45 | 30.7 ± 2.94 | 31.7 ± 0.83 | 30.6 ± 2.96 | |
| La- (mmol/L) | 0.000 | ||||||
| WARDS | 1.01 ± 0.19 | 1.06 ± 0.19 | 4.23 ± 0.75 c | 1.44 ± 0.29 | 5.87 ± 0.46 c | 1.37 ± 0.19 | |
| WT | 0.97 ± 0.30 | 0.98 ± 0.33 | 2.24 ± 0.30 | 1.29 ± 0.28 | 2.23 ± 0.35 | 1.28 ± 0.38 |
Indexes and Blood Gases a
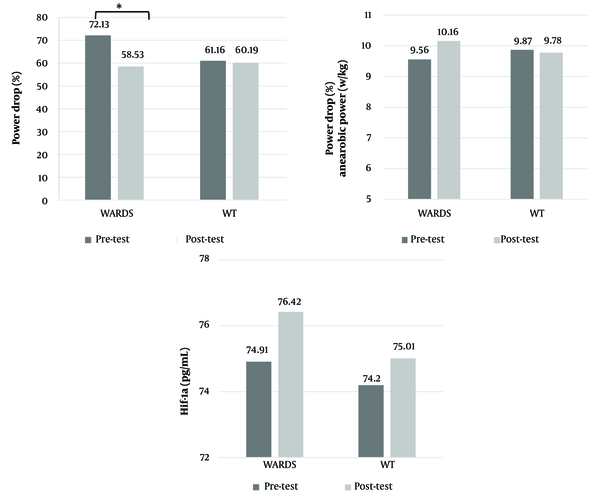
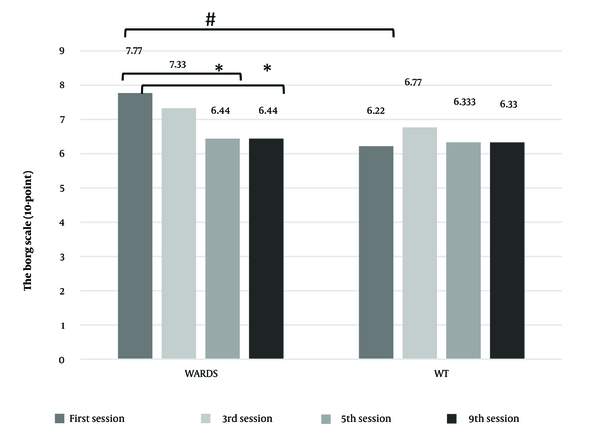
The results for HIF-1α, anaerobic power, FI, and RPE are displayed in Figures 1 and 2. The findings indicate that changes in HIF-1α concentration (P = 0.079) and anaerobic power (P = 0.534) did not exhibit significant intergroup differences. However, significant intergroup differences were observed in FI (P ≤ 0.001) and RPE (P = 0.041).
5. Discussion
The aim of this study was to investigate the effect of weightlifting exercises with ARDS on buffering capacity, HIF-1α, anaerobic power, FI, and RPE in weightlifters. The results indicated that using added dead space in weightlifting exercises enhanced buffering capacity, resulting in a 1.97% increase in HIF-1α levels, a 5.9% increase in anaerobic power, and a 23.2% reduction in FI in the WARDS group. Additionally, the RPE Index during training sessions decreased in the WARDS group, showing no significant difference between the two training groups on the final training day. Athletes and coaches can adopt this low-cost and efficient training method to improve buffering capacity, enhance anaerobic power, and reduce fatigue.
The adaptations resulting from increased mean partial pressure of CO2 during ARDS training and the elevated HCO3⁻ concentration from the carbonic anhydrase reaction (15-17) contribute to delayed acidosis and improved buffering capacity (18), which can further promote anaerobic metabolism (10). La⁻, a potent signaling molecule, induces an increase in the HIF-1α factor (13). In line with our study’s findings, implementing the ARDS training method caused a significant rise in La⁻ levels in the WARDS group, consistent with the 1.97% increase in HIF-1α levels in this group. Factors such as acute exercise, acidosis, oxidative stress, and heat have been shown to activate HIF-1α expression independently of hypoxia (19). Additionally, La⁻ can increase HIF-1α levels even in the presence of oxygen (13).
However, the results of our study contrast with those of Selfridge et al., who reported that increased CO2 responses reduce HIF transcription activity and that low pH conditions facilitate the lysosomal degradation of HIF-α protein (20).
The reason for this difference may lie in the fact that Selfridge et al. examined acute CO2 exposure conditions, whereas in our study, participants trained under high CO2 conditions for 10 weeks, and we measured the adaptations resulting from these conditions. Selfridge also suggested that the acidic pH conditions associated with high CO2 exposure might deprive cells of nutrients, prompting a response in the form of lysosomal degradation of HIF-α protein (20). However, with the observed increase in La⁻ and HCO3⁻ levels in the WARDS group of our study, it seems that enhanced buffering capacity facilitated better H⁺ elimination, thereby preventing the reduction of HIF-1α.
Research has demonstrated that RPE is a valuable tool for prescribing exercise intensity (21). Prescribing RPE-based training programs allows individuals to maintain exercise intensity within a predetermined RPE range, which is closely associated with objective physiological markers of intensity, such as heart rate, oxygen consumption, or blood La⁻ levels (22). Numerous studies have reported a strong correlation between blood La⁻ and RPE during exercise (23, 24). In our study, RPE levels showed significant differences between the two groups only in the initial training sessions, with no differences observed by the seventh and tenth weeks. This suggests that using a mask and tube during training was not perceived as more difficult by participants in the WARDS group. Furthermore, the WARDS group trained under higher CO2 and La⁻ conditions with similar RPE levels and demonstrated superior results in buffering capacity, anaerobic power, and FI.
Our findings align with the results of studies by Danek et al. and López-Cabral et al. (15, 25). In Danek et al.'s study, 11 active individuals performed six 10-second repetitions with four minutes of active recovery over four laboratory sessions. The work done in the mask group (4.4%) and the average HCO3⁻ concentration (6.7%) were higher, with no difference in RPE between groups (15). Similarly, López-Cabral’s study stated that reductions in RPE were associated with increases in La⁻ due to metabolic adaptations during resistance training (25).
However, our results were inconsistent with findings by Miller et al. and Green et al. (22, 26). Miller et al. reported that exogenous La⁻ intake during exercise did not affect RPE levels (26). Additionally, Green et al.'s study found a negative correlation between La⁻ concentration and RPE during cycling exercise, where RPE levels were lower when La⁻ levels were highest (22). In these studies, the exercise duration was much shorter than in our research, and the researchers focused on the immediate response of La⁻ to RPE. In contrast, our study examined the adaptations created over a prolonged training period, which are likely to influence the measured factors differently (27). Players with higher anaerobic power and lower FI are capable of superior performance in high-intensity activities (28). Researchers emphasize that elevated metabolic stress is a key factor in enhancing anaerobic power after training (6). Improved buffering capacity, through increased bicarbonate and its role in compensating for the energy demands of the anaerobic system (29), contributes to elevated La⁻ levels, as observed in this study. Alongside the rise in La⁻ levels, an increase in HIF-1α levels was also noted. Several studies have reported a specific relationship between HIF-1α levels and anaerobic power. It has been demonstrated that the distribution of HIF genotypes in strength and power athletes, such as weightlifters, sprinters, and short-distance swimmers, differs from the general population. Athletes with certain HIF alleles exhibit resistance to hypoxic conditions and possess enhanced glycolysis and angiogenesis capabilities, making them particularly adept at power sports (12).
In studies conducted by Ahmetov et al. (11) and Cieszczyk et al. (12), a positive correlation was observed between the frequency of the HIF-1α allele in weightlifters and their level of success. The frequency of this allele was notably higher in athletes compared to the control group. In the present study, findings from the WARDS group compared to the WT group revealed that an increase in HIF-1α levels (1.97% vs. 1.07%) was accompanied by an increase in anaerobic power (5.9% vs. -0.9%) and a significant decrease in the FI (-23.2% vs. -1.6%).
Both the aforementioned studies involved a large number of athletes and non-athletes to address potential issues of population stratification and assessed HIF-1α distribution through DNA testing and biopsies. While the present study had a smaller sample size, it supports the findings of these larger studies, demonstrating similar trends and validating the relationship between HIF-1α levels, anaerobic power, and reduced fatigue.
5.1. Conclusions
Our study results demonstrated that employing the ARDS training strategy in weightlifting exercises enhanced buffering capacity and, through increased HIF-1α levels, improved anaerobic power while reducing FI, without elevating RPE. Trainers and athletes involved in anaerobic sports activities who aim to enhance performance efficiently and reduce fatigue can benefit from incorporating this training method.
However, our study had limitations. The small sample size introduces potential ambiguities, preventing a robust confirmation of the hypothesized relationship between a specific type of HIF-1α and anaerobic power. It is important to note that athletic performance is a multi-gene trait, and further exploration of other performance-related factors is warranted. Additionally, the findings of this study should be corroborated by future research involving longer durations, elite athletes, and female participants to provide a more comprehensive understanding of the impact of ARDS training on performance.